修回日期: 2004-01-09
接受日期: 2004-02-01
在线出版日期: 2004-05-15
目的: 探讨大鼠同种异体肝细胞经腹腔、脾脏、门静脉移植对药物性急性肝衰竭大鼠的治疗作用.
方法: D-氨基半乳糖诱导大鼠急性肝衰竭后60 h, 分6组进行治疗实验. Ⅰ组: 腹腔内移植2×1010/L肝细胞悬液1 mL; Ⅱ组: 腹腔内注射生理盐水1 mL; Ⅲ组: 经门静脉注射2×1010/L肝细胞悬液1 mL; Ⅳ组: 经门静脉注射生理盐水1 mL; Ⅴ组: 向脾脏内移植2×1010/L肝细胞悬液1 mL; Ⅵ组: 脾内注射生理盐水1 mL. 移植同时各组大鼠肌肉注射环孢霉素A(CsA)10 mg/kg, 观察大鼠的存活率、肝脏功能和肝脏病理变化情况.
结果: Ⅰ组大鼠存活率比Ⅱ组有明显提高(60%对20%, P<0.01), 肝脏功能及肝脏病理明显改善. Ⅲ组大鼠存活率与Ⅳ组无显著差异(20%对13.3%, P>0.05), 肝脏功能及肝脏病理未见改善. Ⅴ组与Ⅵ组比较存活率有提高(47%对20%, P<0.05).
结论: 经腹腔及脾脏移植同种异体肝细胞可明显改善D-gal诱导的肝衰竭大鼠的存活率, 部分改善肝功能及肝脏病理; 经门静脉移植同种异体肝细胞不能改善D-gal诱导的肝衰竭大鼠的存活率、肝脏功能及肝脏病理.
引文著录: 李羽, 白雪帆, 张海, 张岩. 急性肝衰竭大鼠肝细胞移植的实验研究. 世界华人消化杂志 2004; 12(5): 1125-1128
Revised: January 9, 2004
Accepted: February 1, 2004
Published online: May 15, 2004
AIM: To study the effect of allogeneic hepatocytes transplantation (HcT) intraperitoneally, intrasplenically or through vena portae in rats with acute hepatic failure (AHF) induced by D-galactosamine (D-gal).
METHODS: AHF rats were induced by D-gal. HcT was carried out 60h after intoxication, and all rats were divided into six groups: GroupⅠ received 2×1010/L hepatocytes 1 mL intraperitoneally with cyclosporin A (CsA) at 10 mg/kg simultaneously; Group Ⅱ received 1 mL normal saline (NS) intraperitoneally with CsA10 mg/kg; Group Ⅲ received 2×1010/L hepatocytes 1 mL through vena portae; Group Ⅳreceived 1mL NS through vena portae; Group Ⅴreceived 2×1010/L hepatocytes 1 mL intrasplenically; Group Ⅵ received 1 mL NS intrasplenically. After 1 wk the survival rates, liver function and liver histology of all rats were observed.
RESULTS: The survival rate of Group Ⅰ was higher than that of GroupⅡ (60 % vs 20%, P < 0.01), and their liver function and liver histology were obviously improved as compared with GroupⅡ. Similarly, the survival rate of Group Ⅴ was higher than that of Group Ⅵ (47% vs 20%, P < 0.05), and the liver function and liver histology were also improved in GroupⅤas compared with Group Ⅵ. On the other hand, the survival rate of Group Ⅲ was similar to that of GroupⅥ (20% vs 13.3%, P > 0.05), and their liver function and liver histology were also not improved significantly as compared with Group Ⅱ.
CONCLUSION: After HcT intraperitoneally or intrasplenically, the survival rates of AHF rats intoxicated with D-gal are increased, and the liver function and histology are also improved. On the contrary, the survival rate, liver function and liver histology of AHF rats through vena portae HcT are not improved.
- Citation: Li Y, Bai XF, Zhang H, Zhang Y. Hepatocytes transplantation in rats with acute hepatic failure. Shijie Huaren Xiaohua Zazhi 2004; 12(5): 1125-1128
- URL: https://www.wjgnet.com/1009-3079/full/v12/i5/1125.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i5.1125
急性肝功能衰竭患者死亡的主要原因在于大量肝细胞坏死. 目前, 原位肝脏移植是挽救肝衰竭的惟一有效方法, 但由于受到肝脏供源及手术费用等因素的限制, 目前在我国广泛开展肝脏移植以治疗肝衰竭仍面临许多困难. 肝细胞移植作为一种简便有效且对机体影响较小的技术愈来愈受到关注[1-5], 与肝移植相比, 他具有以下优点: (1)一个供体可以给多个受体提供细胞; (2)移植技术相对简单; (3)即使移植失败, 对受体影响也十分微小. 肝细胞移植近年来得到较快发展, 成为继原位肝移植后又一治疗各种肝功能衰竭的有效及有应用前景的方法[6-8]. 我们采用D-氨基半乳糖(D-gal)诱导大鼠肝衰竭, 经门静脉、脾脏及腹腔移植同种异体肝细胞治疗急性肝衰竭大鼠, 拟在观察急性肝衰竭大鼠存活率、肝脏功能及肝脏病理的变化, 为临床开展肝细胞移植提供了新的方法与途径.
IV型胶原酶及DNA酶I购自Sigma公司, D-氨基半乳糖(D-gal)购于重庆医科大学. 成年SD大鼠由第四军医大学实验动物中心提供. 20 g/L戊巴比妥钠溶液30 mg/kg腹腔麻醉, 同时腹腔内注射200 IU肝素钠溶液. 采用原位二步法门静脉胶原酶灌注分离肝细胞, 制成肝细胞混合悬液, 37 ℃恒温水浴孵化10 min, 然后, 将此悬液以100目及200目网过滤, 分装于3-4离心管中, 以500 r/min离心2 min, 弃上清, 以4 ℃Hanks液重悬, 清洗并反复离心3次, 再以4 ℃ Hanks液重悬, 做细胞涂片行苏木精-伊红(HE)染色判断肝实质细胞纯度, 用4 g/L台盼蓝(trypan blue)染色判断细胞活率, 用血细胞计数板检查细胞数量并计算细胞产量.
成年SD大鼠186只, 体质量170±30 g, 清洁级. 180只大鼠腹腔内注射1.6 g/kg的D-gal, 根据肝脏病理及肝功能改变情况制备大鼠急性肝衰竭模型. 另6只大鼠设为正常对照. 肝衰竭动物随机分为6组: Ⅰ组30只, 腹腔内移植浓度为2×1010/L的肝细胞悬液1 mL; Ⅱ组30只, 腹腔注射生理盐水1 mL作为I组的对照组; Ⅲ组 30只, 常规麻醉后切开腹腔, 经门静脉穿刺注射浓度为2×1010/L的肝细胞悬液1 mL; Ⅳ组 30只, 经门静脉注射生理盐水1 mL, 其余处理同III组; Ⅴ组30只, 常规麻醉后切开腹腔, 将脾脏提出体外, 由尾端向脾内注射浓度为2×1010/L的肝细胞悬液1 mL, 结扎脾尾; Ⅵ组 30只作为Ⅴ组的对照, 脾内注射生理盐水1 mL, 其余处理同前. 各鼠在接受移植的同时肌肉注射环孢霉素A(CsA) 10 mg/kg, 每组分取15只用于观察1 wk存活率及组织学检查, 15只用于抽血及组织学检查. 观察各组大鼠在接受移植后的症状表现, 分别于细胞移植后1, 3, 5, 7 d统计各组大鼠存活情况. 于肝细胞移植后l, 3, 7 d, 经腹主动脉抽血, 用全自动生化分析仪测丙氨酸氨基转移酶(ALT), 天冬氨酸氨基转移酶(AST), 总胆红素(TBi), 碱性磷酸酶(ALP). 取6只大鼠作正常对照. 取大鼠肝脏标本, 用40 g/L中性甲醛固定, 按常规HE染色, 观察肝组织学改变.
统计学处理 所有数据以mean±SD 表示, 死亡率比较采用Fisher确切概率计算, 各项肝功能指标比较采用团体t检验.
所有大鼠于药物诱导后逐渐进入严重的肝功能衰竭状态. 最初表现为活动减少, 不思饮食乃至嗜睡、共济失调、昏迷, 部分伴尿失禁、尿色深黄, 口腔、泌尿生殖道出血. 不同处理组大鼠1 wk存活率Ⅰ组为60%(9/15), Ⅱ组为20%(3/15), Ⅰ组比Ⅱ组大鼠存活率明显提高(P<0.01); Ⅲ组为20%(3/15), Ⅳ组为13.3%(2/15), Ⅲ组与Ⅳ组无显著差异(P>0.05); Ⅴ组为47%(7/15), Ⅵ组为20%(3/15), 两组之间差异明显(P<0.05).
各对照组(Ⅱ, Ⅳ, Ⅵ)肝衰竭大鼠ALT, AST, TBi等指标在细胞移植前及细胞移植后1 d无明显差异, 3 d肝功能异常达高峰, 7 d逐步恢复. 统计学分析显示, 在移植肝细胞后1 d, 不同移植方式治疗与对照组间各项指标差异无显著性(P>0.05).至3 d, Ⅰ组与Ⅱ组, Ⅴ组与Ⅵ组比较各项指标均有改善(P<0.05), Ⅲ组与Ⅳ组变化不明显. 7d, 存活鼠肝功能明显恢复, 各组之间无显著差异(表1).
| 分组 | ALT(mkat/L) | AST(mkat/L) | TBi(mmol/L) | ALP(mkat/L) |
| 正常鼠 | 0.7±0.3 | 1.9±0.9 | 1.7±2.3 | 2.3±0.9 |
| 模型鼠1 d Ⅰ | 31.3±10.4 | 40.7±10.9 | 133.4±51.7 | 3.4±0.9 |
| Ⅱ | 32.7±10.4 | 43.2±14.1 | 141.1±47.4 | 4.1±1.0 |
| Ⅲ | 32.8±12.6 | 43.7±9.0 | 137.4±34.4 | 5.3±1.0 |
| Ⅳ | 32.6±8.3 | 46.0±9.2 | 149.7±72.1 | 5.1±1.3 |
| Ⅴ | 32.0±11.4 | 42.0±12.1 | 142.3±68.8 | 4.7±1.1 |
| Ⅵ | 32.5±12.0 | 41.5±13.7 | 151.1±58.7 | 4.9±1.0 |
| 3 d Ⅰ | 14.8±5.2 | 18.8±10.9 | 70.9 ±36.3 | 2.5±1.2 |
| Ⅱ | 37.0±19.4 | 51.6±27.1 | 166.1±68.3 | 4.3±1.7 |
| Ⅲ | 26.9±14.4 | 43.4±20.7 | 164.4±88.4 | 3.8±1.6 |
| Ⅳ | 38.0±15.7 | 51.7±18.8 | 176.0±67.3 | 4.3±1.6 |
| Ⅴ | 14.9±5.5 | 20.7±12.1 | 92.8 ±32.7 | 3.0±1.2 |
| Ⅵ | 36.3±16.9 | 51.3±21.3 | 172.4±40.2 | 4.6±1.8 |
| 7 d Ⅰ | 0.8±0.5 | 1.9±0.9 | 2.5 ±3.1 | 2.2±0.8 |
| Ⅱ | 1.1±0.4 | 2.1±0.5 | 1.8 ±2.2 | 3.1±1.1 |
| Ⅲ | 0.9±0.5 | 2.2±0.6 | 2.5 ±3.8 | 3.1±1.0 |
| Ⅳ | 0.8±0.4 | 2.3±0.8 | 1.9 ±3.1 | 2.7±1.3 |
| Ⅴ | 0.8±0.5 | 2.0±1.0 | 2.0 ±1.5 | 2.1±0.9 |
| Ⅵ | 1.0±0.6 | 2.3±0.8 | 2.1 ±1.8 | 2.9±1.0 |
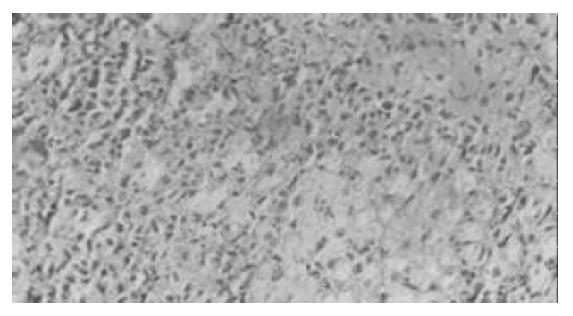
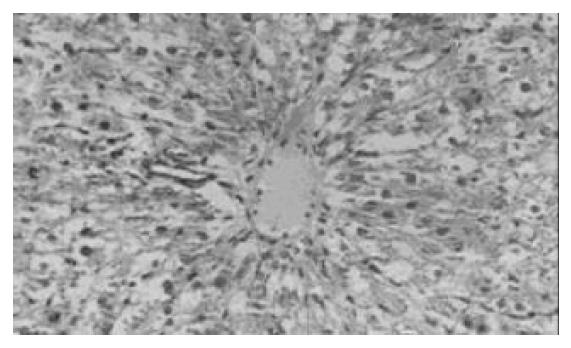
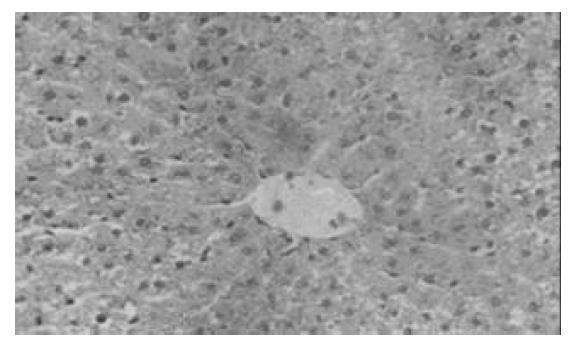
对照组(Ⅱ, Ⅳ, Ⅵ)在肝细胞移植后1, 3 d , 肝脏小叶结构破坏, 肝索紊乱. 肝细胞大片变性坏死, 表现为胞质疏松, 大量空泡, 可见核固缩或碎裂(图1). Ⅰ组与Ⅴ组在移植后3 d, 肝脏组织病变轻于其对照组, 部分标本亦可见肝细胞破坏但较轻, 表现为肝小叶结构尚清楚, 肝细胞变性为主, 坏死灶小, 炎性细胞浸润少由汇管区开始淋巴细胞、中性白细胞等炎性细胞浸润, 坏死区亦见炎性细胞浸润(图2). Ⅴ组细胞移植后3 d, 脾内可见散在的肝细胞或成群的细胞团,多围绕淋巴滤泡分布. 肝细胞可呈圆形、椭圆形或多角形,胞质欠丰富. 可见少量炎性细胞攻击. 7 d脾脏切片内已无法找到形态典型的肝细胞,多为纤维化条索替代(图3). Ⅰ组在移植后1 d, 腹腔内有游离的黄豆大小的黄白色团块及网膜包裹团块, HE染色见肝细胞, 细胞成簇排列, 周围可见普弗细胞, 淋巴细胞等大量炎性细胞浸润; 5 d后腹腔不见游离团块, 仅见个别网膜裹聚团块, HE染色后难见完整肝细胞, 可见淋巴细胞, 肝细胞被纤维组织或肉芽组织替代; 7 d腹腔内无肝细胞, 仅见纤维组织. 细胞移植后7 d, 各组动物肝组织学结构均大致恢复正常.
肝细胞移植可以提供急性肝功能损害的代谢支持, 以便让急性肝衰患者的肝细胞再生到足以支持肝功能或使患者度过急性肝衰的难关而等待原位肝移植, 因而成为目前肝移植研究的热点之一. 目前, 随着肝细胞材料方面研究进展, 如肝细胞的分离纯化及微载体技术的进一步深入[9-21], 动物实验研究水平有了明显的提高, 且国外临床试验已取得较理想的治疗效果[6,22-24]. 肝细胞移植提高生存率的主要机制包括提供暂时的肝功能支持, 以及可通过产生肝细胞生长刺激因子促进残存正常肝脏的再生和功能恢复[25-29]. 研究表明, 植入的肝细胞可帮助机体迅速度过肝衰期, 提高受体的存活率.
肝细胞移植部位的选择非常重要, 因为成功的肝细胞移植需要来自门静脉血的亲肝因子的营养作用才足以维持异位移植肝细胞的功能[30]. 脾实质是较理想的移植部位, 脾内移植的肝细胞具有良好的生理功能和长期存活率[31-35], 肝脏本身也因门脉内含高浓度的嗜肝细胞因子和局部生长因子, 成为移植肝细胞生存的理想环境[36-37] , 腹腔内移植因具有操作便利、营养物质及代谢产物交换表面积大等特点[38]也常被选用. 我们采用门静脉、脾脏和腹腔3种途径进行同种异体肝细胞移植, 结果证实同种异体肝细胞经腹腔、脾脏移植可显著改善D-gal诱导的急性肝衰竭大鼠的存活率, 治疗效果明显, 与相关文献[28, 35, 38-39]报道一致. 肝脏病理改变与生化指标变化基本平行, 细胞移植后1 d, 各治疗组与对照组比较, 肝脏病理无明显改善. 移植后3 d, 脾脏及腹腔移植组肝脏组织学变化明显好于其对照组, 移植肝细胞7 d, 各组大鼠肝组织均得到明显恢复.
本结果还表明, 同种异体肝细胞经门静脉移植不能提高D-gal诱导的急性肝衰竭大鼠的存活率, 其疗效较差, 与临床治疗效果存在一定差异[22,24]. 肝细胞移植后实验鼠死亡率较高, 可能系由于实验大鼠个体小, 门静脉内径窄, 穿刺行肝细胞移植难度较高, 移植细胞数量有限, 且易发生门静脉出血、门静脉栓塞至肝脏急性缺血、坏死等并发症所至. 故有待较大个体肝衰竭动物的实验研究验证.
采用D-gal诱导急性肝衰竭大鼠模型, 药物注射后2 d, 肝脏生化指标ALT, AST, TBi, ALP出现明显变化, 至5 d该变化达到高峰. 7 d, 各组大鼠肝脏功能均大致恢复正常, 同时肝脏的组织学变化与其生化指标改变基本平行. 提示该方法虽可较好模拟临床急性肝衰竭患者的病理生理改变, 但仍存在模型动物生存时间短及稳定性较差的问题.
总之, 运用肝细胞移植治疗急性肝功能衰竭的效果是肯定的, 且会随着相关研究的深入而取得更加明显的疗效, 同时肝细胞移植部位及方式、细胞种类的选择, 细胞体外培养时间等问题也亟待解决. 本研究证实不同移植途径的治疗效果存在一定的差异, 经脾脏及腹腔移植肝细胞治疗急性肝衰竭疗效明显, 为临床开展肝细胞移植研究提供了新的资料
| 1. | Di Campli C, Gasbarrini G, Gasbarrini A. Review article: a medicine based on cell transplantation -- is there a future for treating liver diseases? Aliment Pharmacol Ther. 2003;18:473-480. [PubMed] [DOI] |
| 2. | Di Campli C, Nestola M, Piscaglia AC, Santoliquido A, Gasbarrini G, Pola P, Gasbarrini A. Cell-based therapy for liver diseases. Eur Rev Med Pharmacol Sci. 2003;7:41-44. [PubMed] |
| 3. | Leckel K, Blaheta RA, Markus BH. [State of hepatocyte transplantation: a risk or a chance?]. Zentralbl Chir. 2003;128:283-290. [PubMed] [DOI] |
| 4. | Gupta S. Hepatocyte transplantation. J Gastroenterol Hepatol. 2002;17 Suppl 3:S287-S293. [PubMed] [DOI] |
| 5. | Gupta S, Chowdhury JR. Therapeutic potential of hepatocyte transplantation. Semin Cell Dev Biol. 2002;13:439-446. [PubMed] [DOI] |
| 6. | Ng VL, Alonso M, Bezerra JA. Hepatocyte transplantation. Advancing biology and treating children. Clin Liver Dis. 2000;4:929-945, vii. [PubMed] [DOI] |
| 7. | Nakamura J, Okamoto T, Schumacher IK, Tabei I, Chowdhury NR, Chowdhury JR, Fox IJ. Treatment of surgically induced acute liver failure by transplantation of conditionally immortalized hepatocytes. Transplantation. 1997;63:1541-1547. [PubMed] [DOI] |
| 8. | Maruyama M, Totsugawa T, Kunieda T, Okitsu T, Shibata N, Takesue M, Kurabayashi Y, Oshita M, Nakaji S, Kodama M. Hepatocyte isolation and transplantation in the pig. Cell Transplant. 2003;12:593-598. [PubMed] [DOI] |
| 9. | Ambrosino G, Varotto S, Basso SM, Cecchetto A, Carraro P, Naso A, De Silvestro G, Plebani M, Abatangelo G, Donato D. Hepatocyte transplantation in the treatment of acute liver failure: microencapsulated hepatocytes versus hepatocytes attached to an autologous biomatrix. Cell Transplant. 2003;12:43-49. [PubMed] [DOI] |
| 10. | Mitry RR, Hughes RD, Dhawan A. Progress in human hepatocytes: isolation, culture & cryopreservation. Semin Cell Dev Biol. 2002;13:463-467. [PubMed] [DOI] |
| 11. | Aoki T, Umehara Y, Ferraresso C, Sugiyama N, Middleton Y, Avital I, Inderbitzin D, Demetriou AA, Rozga J. Intrasplenic transplantation of encapsulated cells: a novel approach to cell therapy. Cell Transplant. 2002;11:553-561. [PubMed] |
| 22. | Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, Bernard Otte J, Evrard V, Latinne D, Vincent MF. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735-738. [PubMed] [DOI] |
| 23. | Nagata H, Ito M, Cai J, Edge AS, Platt JL, Fox IJ. Treatment of cirrhosis and liver failure in rats by hepatocyte xenotransplantation. Gastroenterology. 2003;124:422-431. [PubMed] [DOI] |
| 24. | Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, Fox IJ. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111:1262-1267. [PubMed] [DOI] |
| 25. | Strain AJ, Neuberger JM. A bioartificial liver--state of the art. Science. 2002;295:1005-1009. [PubMed] [DOI] |
| 26. | Benoist S, Sarkis R, Chafaï N, Barbu V, Honiger J, Lakehal F, Becquemont L, Baudrimont M, Capeau J, Housset C. Survival and differentiation of porcine hepatocytes encapsulated by semiautomatic device and allotransplanted in large number without immunosuppression. J Hepatol. 2001;35:208-216. [PubMed] [DOI] |
| 27. | Boudjema K, Bachellier P, Wolf P, Tempé JD, Jaeck D. Auxiliary liver transplantation and bioartificial bridging procedures in treatment of acute liver failure. World J Surg. 2002;26:264-274. [PubMed] [DOI] |
| 28. | Watanabe H, Sumi S, Kitamura Y, Nio Y, Higami T. Immunohistochemical analysis of vascular endothelial growth factor and hepatocyte growth factor, and their receptors, in transplanted islets in rats. Surg Today. 2003;33:854-860. [PubMed] [DOI] |
| 29. | Hodgson H. Liver cells: biology to therapeutics. Clin Med (Lond). 2003;3:161-165. [PubMed] [DOI] |
| 30. | Laconi E, Laconi S. Principles of hepatocyte repopulation. Semin Cell Dev Biol. 2002;13:433-438. [PubMed] [DOI] |
| 31. | Kaufmann PM, Kneser U, Fiegel HC, Pollok JM, Kluth D, Izbicki JR, Herbst H, Rogiers X. Is there an optimal concentration of cotransplanted islets of Langerhans for stimulation of hepatocytes in three dimensional matrices? Transplantation. 1999;68:272-279. [PubMed] [DOI] |
| 32. | Rust C, Gores GJ. Hepatocyte transplantation in acute liver failure: a new therapeutic option for the next millennium? Liver Transpl. 2000;6:41-43. [PubMed] [DOI] |
| 33. | Nagata H, Ito M, Shirota C, Edge A, McCowan TC, Fox IJ. Route of hepatocyte delivery affects hepatocyte engraftment in the spleen. Transplantation. 2003;76:732-734. [PubMed] [DOI] |
| 34. | Ba MC, Zhou XD, Chen JS, Liu L. Proliferation of retrorsine-treated SD rat immortalized hepatocytes after intrasplenic transplantation. Diyi Junyi Daxue Xuebao. 2003;23:546-548, 552. [PubMed] |
| 35. | Ahmad TA, Fujioka H, Eguchi S, Yanaga K, Kamohara Y, Furui J, Kanematsu T. Long-term effect of hepatocyte transplantation on fulminant hepatic failure in rats. Hepatogastroenterology. 2003;50:467-471. [PubMed] |
| 36. | Gupta S, Rajvanshi P, Lee CD. Integration of transplanted hepatocytes into host liver plates demonstrated with dipeptidyl peptidase IV-deficient rats. Proc Natl Acad Sci USA. 1995;92:5860-5864. [PubMed] [DOI] |
| 37. | Schneider A, Attaran M, Gratz KF, Bleck JS, Winkler M, Manns MP, Ott M. Intraportal infusion of 99mtechnetium-macro-aggregrated albumin particles and hepatocytes in rabbits: assessment of shunting and portal hemodynamic changes. Transplantation. 2003;75:296-302. [PubMed] [DOI] |
| 38. | Selden C, Casbard A, Themis M, Hodgson HJ. Characterization of long-term survival of syngeneic hepatocytes in rat peritoneum. Cell Transplant. 2003;12:569-578. [PubMed] [DOI] |
| 39. | Hamazaki K, Doi Y, Koide N. Microencapsulated multicellular spheroid of rat hepatocytes transplanted intraperitoneally after 90% hepatectomy. Hepatogastroenterology. 2002;49:1514-1516. [PubMed] |