修回日期: 2003-09-25
接受日期: 2003-11-06
在线出版日期: 2004-03-15
目的: 研究MGMT, hMLH1 和hMSH2 在慢性胰腺炎和胰腺癌组织中表达特征及其临床病理意义.
方法: 胰腺癌(n = 51)和慢性胰腺炎(n = 10)手术切除标本经 40 g/L中性甲醛固定后常规制作石蜡包埋切片, MGMT, hMLH1 和hMSH2表达均采用常规ABC免疫组化法.
结果: 胰腺癌MGMT, hMLH1 和hMSH2表达阳性率(39.2%, 45.1%和50.9%)及其评分(1.8±1.4, 1.7±1.6和1.9±1.7)明显低于慢性胰腺炎阳性率(100.0%, 100.0%和90.0%)及其评分(3.8±0.8, 3.8±1.0和3.5±0.9), 均有显著或高度显著性差异(P<0.05或P<0.01); 转移病例三者表达阳性率及其评分较明显低于未转移病例, 但均无显著性差异(P<0.05); 三者表达与胰腺癌其他临床病理特征无明显关系.
结论: MGMT, hMLH1 或hMSH2表达与胰腺癌发生及进展密切相关, 均具有抑制胰腺癌发生及进展的作用.
引文著录: 杨竹林, 邓星辉, 李永国, 钟德玝, 苗雄鹰. 胰腺癌组织MGMT, hMLH1和hMSH2的表达意义. 世界华人消化杂志 2004; 12(3): 669-672
Revised: September 25, 2003
Accepted: November 6, 2003
Published online: March 15, 2004
AIM: To study the expression of MGMT, hMLH1 and hMSH2 and their clinicopathological significances in the tissues of chronic pancreatitis and pancreatic adenocarcinoma.
METHODS: The expression levels of MGMT, hMLH1 and hMSH2 were assayed by immunohistochemical method of avidin-biotin complex on the formalin-fixed and routinely paraffin-embedded sections of surgical resected specimen with chronic pancreatitis (n = 10) and pancreatic carcinoma (n = 51).
RESULTS: The positive rates and the scores of MGMT, hMLH1 and hMSH2 were significantly higher in chroinic pancreatitis than those of pancreatic carcinoma (MGMT: 100.0% vs 39.2%,3.8±0.8 vs 1.8±1.4; hMLH1: 100.0% vs 45.1%, 3.8±1.0 vs 1.7±1.6; hMSH2: 90.0% vs 50.9%, 3.5±0.9 vs 1.9±1.7). The positive rates and the scores of MGMT, hMLH1 and hMSH2 were significantly higher in well-differentiated adenocarcinomas than those of poorly differentiated adenocarcinomas (P < 0.05 or P < 0.01). The positive rates and the scores of MGMT, hMLH1 and hMSH2 were higher in metastasis-free cases than those of ones with metastasis, but no statistic difference was found (P>0.05). There was also no difference among the expression of three proteins and the other clinicopathological characteristics of pancreatic carcinoma.
CONCLUSION: The expression of MGMT, hMLH1 or hMSH2 may be related to the carcinogenesis and progression, and have inhibifory effects on the carcinogenesis and progression of pancreatic carcinoma.
- Citation: Yang ZL, Deng XH, Li YG, Zhong DW, Miao XY. Expression of MGMT, hMLH1 and hMSH2 and its clinopathological significance in pancreatic carcinoma tissues. Shijie Huaren Xiaohua Zazhi 2004; 12(3): 669-672
- URL: https://www.wjgnet.com/1009-3079/full/v12/i3/669.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i3.669
烷化剂是环境中普通存在的致DNA损伤的一类物质, O6-甲基鸟嘌呤-DNA甲基转移酶(O6-methylguanine-DNA methyltransferase, MGMT)是机体修复烷基化合物的关键酶, 对抗烷化剂造成的DNA损伤, 在肿瘤发生及化疗中有十分重要意义[1-11]. 错配修复是细胞复制后一种修复机制, 起着维持DNA复制保真度和控制基因突变的作用, 目前至少发现6个参与错配修复功能的基因, 其中最主要的有hMLH1和hMSH2, 该系统中任一基因突变都会导致细胞错配修复功能缺陷, 表现为复制错误或微卫星不稳定, 因而与肿瘤发生密切相关[4-8,12-23]. 我们应用免疫组化方法研究胰腺癌和慢性胰腺炎组织中MGMT, hMLH1 和hMSH2表达特征及其临床病理意义.
我院及湘雅医院胰腺癌手术切除标本51例, 男38例, 女13例, 年龄21-73(51±17岁); 均为胰腺导管上皮癌, 包括高分化腺癌20例, 中分化腺癌12例和低分化腺癌19例; 临床和(或)病理证实发生胰腺外转移(包括区域淋巴结、网膜、邻近组织器官等) 35例(68.6%). 另慢性胰腺炎手术切除标本10例, 男7例, 女3例, 年龄35-55(44±10 岁). 标本经固定后常规制作石蜡包埋切片, 切片厚4 m. HE染色复述病理组织学特征, 其他切片行免疫组化染色. 兔抗人MGMT, hMLH1 和hMSH2多克隆抗体, 生物素标记羊抗兔lGg, ABC试剂及DAB-Hcl显色试剂盒均购自武汉博士德公司.
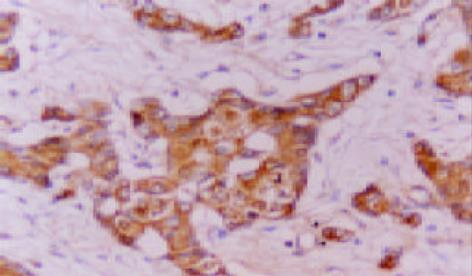
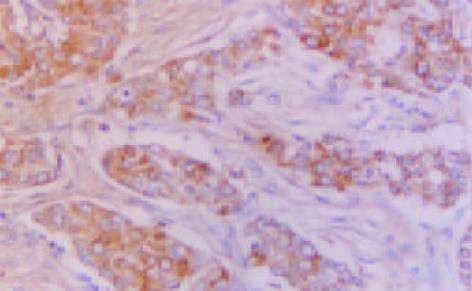
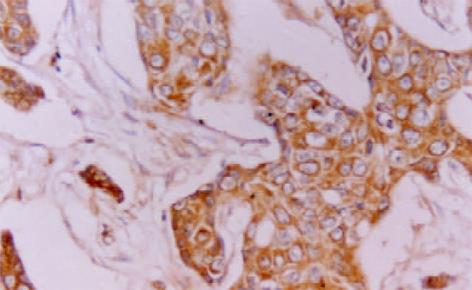
MGMT, hMLH1 和hMSH2表达均为常规 ABC 免疫组化法, 胞质内出现明显棕黄色颗粒者为阳性细胞. 参照三者评分标准[6,8,11,23,25]将细胞染色强度评分(无, 0分; 弱, 1分; 中度, 2分; 强度, 3分)和阳性细胞率评分(小于5%, 0分; 5-10%, 1分; 10%-20%, 2分; 20%-50%, 3分; 大于50%, 4分)之和为该病例评分值, 将评分值小于或等于2分定为阴性病例, 大于2分定为阳性病例. 以博士德公司提供的阳性切片作为染色的阳性对照, 以0.01 mol/L PBS液(pH7.4)替代-抗作为每次染色的阴性或替代对照.
统计学处理 采用SPSSS10.0统计软件包进行t检验, x2检验或Fischer精确概率法, 检验水准=0.05.
MGMT, hMLH1 和hMSH2免疫反应阳性物质主要定位于胞质, 部分病例偶见胞核着色(图1-3). 三者在癌组织中分布呈较明显异质性, 同一切片不同视野阳性细胞率及着色程度可有较明显不同.胰腺癌51例MGMT, hMLH1和hMSH2阳性病例分别为20(39.2%)、23(45.1%)和26(50.9%)例, 其评分值分别1.8±1.4, 1.7±1.6和1.9±1.7; 慢性胰腺炎10例仅hMSH2 1例阴性表达, 其评分值分别为3.8±0.8、3.8±1.0和3.5±0.9, 胰腺癌三者表达阳性率及其评分均明显低于慢性胰腺炎 (P<0.05或P<0.01). 高分化腺癌三者表达阳性率及其评分明显高于低分化腺癌(P<0.05或P<0.01); 未转移胰腺癌病例三者表达阳性率及其评分较明显高于转移病例, 但均无统计学差异(P>0.05, 表1). 胰腺癌其他临床病理特征与三者表达均无明显关系(P>0.05).
人MGMT基因定位于10q26, 编码由207个氨基酸组成的蛋白质, 活性位点在145位半胱氨酸残基. 生理条件下MGMT是一种含磷蛋白, 磷酸化可抑制其活性, 蛋白激酶C、酪氨酸激酶Ⅱ等可使其磷酸化, 影响其功能, 但该酶磷酸化后可抵抗蛋白酶对他的消化而得以保存活性, 碱性磷酸酶可使其去磷酸化而恢复活性[5-11,25].MGMT在正常及大多数肿瘤组织中均有表达(称为Mer+表型), 约5%肿瘤组织和20%肿瘤细胞株检测不到MGMT活性(称为Mer-表型)[1-2]. MGMT表达与许多肿瘤的发生及肿瘤耐药性密切相关, 如食管癌, 乳腺癌、胃癌、肝癌等[4-11]. MGMT表达具有组织及细胞类型特异性, Mer-细胞内MGMT基因本身极少发生突变、缺失、重排等改变, 其mRNA水平和蛋白水平是一致的. 但关于其在转录水平的精确调控机制尚不清楚, 可能与启动子区域CpG岛甲基化有关[1-3]. MGMT基因调节失活增加了靶细胞对烷化剂损伤的易感性, 在早期肿瘤发生中起重要作用[1,3,11]. 烷化剂与胸腺嘧啶错配, 发生G: C→A: T突变, 可导致癌基因激活和抑癌基因失活, 进一步发展为肿瘤[4-11]. 我们发现胰腺癌MGMT表达阳性率及其评分明显地低于慢性胰腺炎, 高分化腺癌MGMT表达阳性率及其评分也明显地高于低分化腺癌, 与国外文献[4-11]报道较一致. 说明MGMT在抑制胰腺癌发生和进展方面可能起重要作用, 其机制除与MGMT本身生物学作用有关外仍需进一步研究.
hMLH1基因位于染色体3p21, 与酿酒酵母MLH1高度同源. 已证实约30%HNPCC(遗传性非息肉性大肠癌)病因与hMLH1突变有关[13]. 研究发现HCT116细胞(结肠肿瘤细胞株)中有hMLH1缺陷, 表现为错配修复功能缺陷和微卫星序列不稳定, 且对MNNG耐受. 微细胞融合技术将3号染色体上野生型hMLH1基因导入HCT116细胞中, 该细胞株恢复了错配. hMLH1高度甲基化是引起转微卫星不稳定的重要原因, 在散发性大肠癌、子宫内膜癌和胃癌等肿瘤中均有发现[5,7,13-24]. hMSH2是第一个被分离的人类错配修复基因, 位于染色体2p22-22或2p16上. 研究发现约60% HNPCC与hMSH2突变有关, 突变无明显热点, 但相当多突变为缺失性突变和移码突变形成新的终止密码, 产生载短蛋白[13]. 在其他家族性结肠癌综合征中也发现有hMSH2突变(如Muir-Totte综合征), 在散发性结肠癌、子宫内膜癌、胃癌、肝癌、胆管癌、膀胱癌、黑色素瘤等恶性肿瘤中发现错配修复蛋白功能的缺陷, 证实有hMSH2突变[5,7,13-24]. 现已证实hMSH2以复合蛋白质的形式与错配因子进行结合, 这种错配结合因子是由两种不同蛋白质组成的二聚体, 能特异性地识别并结合错配的DNA序列[5,7]. 故DNA错配修复系统缺陷在许多肿瘤的发生及进展过程中起重要作用, 对大肠癌发生发展的影响作用研究尤为深入[5,7]. 我们发现胰腺癌hMLH1, hMSH2表达阳性率及其评分明显低于慢性胰腺炎, 高分化腺癌二者表达阳性率及其评分也明显高于低分化腺癌. 说明hMLH1和hMSH2表达水平与胰腺癌发生和分化程度有关, 二者表达可抑制胰腺癌发生和进展. 目前, 胰腺癌发病在增加[26-27], 早期诊断困难[28-33], 临床治疗仍不满意[34-38], 受到关注.
我们发现未转移癌MGMT, hMLH1和hMSH2表达阳性率及其评分较明显高于转移癌, 但均无显著性差异, 可能与病例数少有关, 如要确证三者表达与抑制胰腺癌转移发生有关仍需积累研究病例和进行更深入研究. 研究发现MGMT, hMLH1和hMSH2表达与一些恶性肿瘤预后有关, 绝大多数文献显示MGMT1和hMSH2低表达的恶性肿瘤预后明显差于高表达者, 而hMLH1低表达者预后较高表达者好[5,7,9,12,19,22-23].
编辑: N/A
| 1. | Esteller M. Cancer epigenetics: DNA methylation and chromatin alterations in human cancer. Adv Exp Med Biol. 2003;532:39-49. [PubMed] [DOI] |
| 2. | Esteller M, Herman JG. Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. J Pathol. 2002;196:1-7. [PubMed] [DOI] |
| 3. | Nephew KP, Huang TH. Epigenetic gene silencing in cancer initiation and progression. Cancer Lett. 2003;190:125-133. [PubMed] [DOI] |
| 4. | Oue N, Sentani K, Yokozaki H, Kitadai Y, Ito R, Yasui W. Promoter methylation status of the DNA repair genes hMLH1 and MGMT in gastric carcinoma and metaplastic mucosa. Pathobiology. 2001;69:143-149. [PubMed] [DOI] |
| 5. | Kitajima Y, Miyazaki K, Matsukura S, Tanaka M, Sekiguchi M. Loss of expression of DNA repair enzymes MGMT, hMLH1, and hMSH2 during tumor progression in gastric cancer. Gastric Cancer. 2003;6:86-95. [PubMed] |
| 6. | Matsukura S, Miyazaki K, Yakushiji H, Ogawa A, Chen Y, Sekiguchi M. Combined loss of expression of O6-methylguanine-DNA methyltransferase and hMLH1 accelerates progression of hepatocellular carcinoma. J Surg Oncol. 2003;82:194-200. [PubMed] [DOI] |
| 7. | Kohya N, Miyazaki K, Matsukura S, Yakushiji H, Kitajima Y, Kitahara K, Fukuhara M, Nakabeppu Y, Sekiguchi M. Deficient expression of O(6)-methylguanine-DNA methyltransferase combined with mismatch-repair proteins hMLH1 and hMSH2 is related to poor prognosis in human biliary tract carcinoma. Ann Surg Oncol. 2002;9:371-379. [PubMed] |
| 8. | Ma S, Egyházi S, Ringborg U, Hansson J. Immunohistochemical analysis of DNA mismatch repair protein and O6-methylguanine-DNA methyltransferase in melanoma metastases in relation to clinical response to DTIC-based chemotherapy. Oncol Rep. 2002;9:1015-1019. [PubMed] [DOI] |
| 9. | Matsukura S, Miyazaki K, Yakushiji H, Ogawa A, Harimaya K, Nakabeppu Y, Sekiguchi M. Expression and prognostic significance of O6-methylguanine-DNA methyltransferase in hepatocellular, gastric, and breast cancers. Ann Surg Oncol. 2001;8:807-816. [PubMed] [DOI] |
| 10. | Kim SH, Bae SI, Lee HS, Kim WH. Alteration of O6-methylguanine-DNA methyltransferase in colorectal neoplasms in sporadic and familial adenomatous polyposis patients. Mol Carcinog. 2003;37:32-38. [PubMed] [DOI] |
| 11. | Cayre A, Penault-Llorca F, De Latour M, Rolhion C, Feillel V, Ferrière JP, Kwiatkowski F, Finat-Duclos F, Verrelle P. O(6)-methylguanine-DNA methyl transferase gene expression and prognosis in breast carcinoma. Int J Oncol. 2002;21:1125-1131. [PubMed] |
| 12. | Taubert HW, Bartel F, Kappler M, Schuster K, Meye A, Lautenschläger C, Thamm-Mücke B, Bache M, Schmidt H, Holzhausen HJ. Reduced expression of hMSH2 protein is correlated to poor survival for soft tissue sarcoma patients. Cancer. 2003;97:2273-2278. [PubMed] [DOI] |
| 13. | Scartozzi M, Bianchi F, Rosati S, Galizia E, Antolini A, Loretelli C, Piga A, Bearzi I, Cellerino R, Porfiri E. Mutations of hMLH1 and hMSH2 in patients with suspected hereditary nonpolyposis colorectal cancer: correlation with microsatellite instability and abnormalities of mismatch repair protein expression. J Clin Oncol. 2002;20:1203-1208. [PubMed] [DOI] |
| 14. | Wang L, Bani-Hani A, Montoya DP, Roche PC, Thibodeau SN, Burgart LJ, Roberts LR. hMLH1 and hMSH2 expression in human hepatocellular carcinoma. Int J Oncol. 2001;19:567-570. [PubMed] [DOI] |
| 15. | Nunn J, Nagini S, Risk JM, Prime W, Maloney P, Liloglou T, Jones AS, Rogers SR, Gosney JR, Woolgar J. Allelic imbalance at the DNA mismatch repair loci, hMSH2, hMLH1, hPMS1, hPMS2 and hMSH3, in squamous cell carcinoma of the head and neck. Oral Oncol. 2003;39:115-129. [PubMed] [DOI] |
| 16. | Peiró G, Diebold J, Lohse P, Ruebsamen H, Lohse P, Baretton GB, Löhrs U. Microsatellite instability, loss of heterozygosity, and loss of hMLH1 and hMSH2 protein expression in endometrial carcinoma. Hum Pathol. 2002;33:347-354. [PubMed] [DOI] |
| 17. | Scartozzi M, De Nictolis M, Galizia E, Carassai P, Bianchi F, Berardi R, Gesuita R, Piga A, Cellerino R, Porfiri E. Loss of hMLH1 expression correlates with improved survival in stage III-IV ovarian cancer patients. Eur J Cancer. 2003;39:1144-1149. [PubMed] [DOI] |
| 18. | Kassem HS, Varley JM, Hamam SM, Margison GP. Immunohistochemical analysis of expression and allelotype of mismatch repair genes (hMLH1 and hMSH2) in bladder cancer. Br J Cancer. 2001;84:321-328. [PubMed] [DOI] |
| 19. | Jansson A, Arbman G, Zhang H, Sun XF. Combined deficiency of hMLH1, hMSH2, hMSH3 and hMSH6 is an independent prognostic factor in colorectal cancer. Int J Oncol. 2003;22:41-49. [PubMed] |
| 20. | Yeh CC, Lee C, Dahiya R. DNA mismatch repair enzyme activity and gene expression in prostate cancer. Biochem Biophys Res Commun. 2001;285:409-413. [PubMed] [DOI] |
| 21. | Idikio HA. Expression of DNA mismatch repair proteins hMSH2 and hMLH1 and the cyclin G1 inhibitor, p21(waf1/cip1) in pediatric tumors: correlation with response to therapy. Oncol Rep. 2001;8:965-971. [PubMed] |
| 22. | Wani Y, Notohara K, Tsukayama C, Okada S. Reduced expression of hMLH1 and hMSH2 gene products in high-grade hepatocellular carcinoma. Acta Med Okayama. 2001;55:65-71. [PubMed] |
| 23. | Catto JW, Xinarianos G, Burton JL, Meuth M, Hamdy FC. Differential expression of hMLH1 and hMSH2 is related to bladder cancer grade, stage and prognosis but not microsatellite instability. Int J Cancer. 2003;105:484-490. [PubMed] [DOI] |
| 24. | Strom SS, Spitz MR, Yamamura Y, Babaian RJ, Scardino PT, Wei Q. Reduced expression of hMSH2 and hMLH1 and risk of prostate cancer: a case-control study. Prostate. 2001;47:269-275. [PubMed] [DOI] |
| 25. | Dinçer Y, Akçay T, Tortum OB, Alademir Z, Onen S, Doğusoy G. Evaluation of O6-methylguanine DNA methyltransferase activity in patients with gastric cancer. Oncol Res. 2003;13:205-209. [PubMed] |
| 26. | Hua Z, Zhang YC, Hu XM, Jia ZG. Loss of DPC4 expression and its correlation with clinicopathological parameters in pancreatic carcinoma. World J Gastroenterol. 2003;9:2764-2767. [PubMed] [DOI] |
| 27. | Wu GS, Zou SQ, Liu ZR, Wang DY. Bile from a patient with anomalous pancreaticobiliary ductal union promotes the proliferation of human cholangiocarcinoma cells via COX-2 pathway. World J Gastroenterol. 2003;9:1094-1097. [PubMed] [DOI] |
| 28. | Dong WG, Sun XM, Yu BP, Luo HS, Yu JP. Role of VEGF and CD44v6 in differentiating benign from malignant ascites. World J Gastroenterol. 2003;9:2596-2600. [PubMed] |
| 29. | Sun XM, Dong WG, Yu BP, Luo HS, Yu JP. Detection of type IV collagenase activity in malignant ascites. World J Gastroenterol. 2003;9:2592-2595. [PubMed] |
| 30. | He YC, Peng W, Qiao JG, Cao J, Chen JW. Relationship between nuclear morphometry, DNA content and resectability of pancreatic cancer. World J Gastroenterol. 2003;9:1863-1865. [PubMed] [DOI] |
| 31. | Tan ZJ, Hu XG, Cao GS, Tang Y. Analysis of gene expression profile of pancreatic carcinoma using cDNA microarray. World J Gastroenterol. 2003;9:818-823. [PubMed] [DOI] |
| 32. | Zheng M, Liu LX, Zhu AL, Qi SY, Jiang HC, Xiao ZY. K-ras gene mutation in the diagnosis of ultrasound guided fine-needle biopsy of pancreatic masses. World J Gastroenterol. 2003;9:188-191. [PubMed] [DOI] |
| 33. | Yao GY, Zhou JL, Lai MD, Chen XQ, Chen PH. Neuroendocrine markers in adenocarcinomas: an investigation of 356 cases. World J Gastroenterol. 2003;9:858-861. [PubMed] [DOI] |
| 34. | Bai YR, Wu GH, Guo WJ, Wu XD, Yao Y, Chen Y, Zhou RH, Lu DQ. Intensity modulated radiation therapy and chemotherapy for locally advanced pancreatic cancer: results of feasibility study. World J Gastroenterol. 2003;9:2561-2564. [PubMed] |
| 35. | Zhou JH, Zhang HM, Chen Q, Han DD, Pei F, Zhang LS, Yang DT. Relationship between telomerase activity and its subunit expression and inhibitory effect of antisense hTR on pancreatic carcinoma. World J Gastroenterol. 2003;9:1808-1814. [PubMed] [DOI] |
| 36. | He P, Shi JS, Chen WK, Wang ZR, Ren H, Li H. Multivariate statistical analysis of clinicopathologic factors influencing survival of patients with bile duct carcinoma. World J Gastroenterol. 2002;8:943-946. [PubMed] [DOI] |
| 37. | Tang ZH, Qiu WH, Wu GS, Yang XP, Zou SQ, Qiu FZ. The immunotherapeutic effect of dendritic cells vaccine modified with interleukin-18 gene and tumor cell lysate on mice with pancreatic carcinoma. World J Gastroenterol. 2002;8:908-912. [PubMed] [DOI] |
| 38. | Liu B, Staren E, Iwamura T, Appert H, Howard J. Taxotere resistance in SUIT Taxotere resistance in pancreatic carcinoma cell line SUIT 2 and its sublines. World J Gastroenterol. 2001;7:855-859. [PubMed] [DOI] |