修回日期: 2003-10-25
接受日期: 2003-11-13
在线出版日期: 2004-03-15
目的: 观察在肠内营养(EN)条件下不同剂量生长激素(GH)对短肠大鼠残存肠道代偿性增生和吸收功能的影响.
方法: 40只SD♂鼠, 切除85%以上小肠制成短肠综合征(SBS)模型, 随机分成假手术组(n = 8)、SBS组(n = 8)、大剂量GH(H-GH)组(n = 8)、中剂量GH(M-GH)组(n = 8)和小剂量GH(L-GH)组(n = 8). GH注射剂量: H-GH组每日7.5 IU/kg, M-GH组每日3.75 IU/kg, L-GH组每日1.88 IU/kg, 从术后2-15 d每日2次皮下注射GH; 假手术组和SBS组注射等量生理盐水. 术后16 d取残留小肠、结肠作组织学检查, 测血浆血糖、氨基酸、胰岛素样生长因子-1(IGF-1)和免疫组化法测定肝PCNA.
结果: 除假手术组外, 其余各组术后体质量均明显下降, H-GH组体质量下降幅度最小, 与SBS组相比存在显著差异(36±4.4 g vs 94±10.0 g, P<0.05). 各组术后空、回肠长度无显著差异, 但各组空、回肠黏膜厚度差异显著, H-GH组 和M-GH组显著大于SBS组(分别为997±65.9 m, 752±79.3 m和974±67.6 m, 788±75.1 m vs 776±61.0 m, 664±64.0 m, P<0.05), 同样, 各组空、回肠肠壁厚度也差异显著, H-GH组 和M-GH组也显著大于SBS组(分别为1142±65.4 m, 884±91.2 m和1145± 78.7 m, 895±95.6 m vs 848±194.7 m, 776±57.5 m, P<0.05), 但H-GH组 和M-GH组的空、回肠黏膜和肠壁厚度均无显著差异. 能全力推注1.5 h后各组血氨基酸、葡萄糖浓度无显著差异. 各组血IGF-1浓度和肝PCNA无显著差异.
结论: GH可减缓体质量下降幅度; 能促进肠黏膜代偿性增生, 但其促进增生的作用并不始终随GH的剂量增加而增加; 未促进葡萄糖和氨基酸吸收.
引文著录: 苏震东, 秦环龙. 生长激素对短肠综合征鼠残存肠道代偿及吸收功能的影响. 世界华人消化杂志 2004; 12(3): 646-649
Revised: October 25, 2003
Accepted: November 13, 2003
Published online: March 15, 2004
AIM: To evaluate the effects of growth hormone (GH) on the residual small intestine of rats with short bowel syndrome (SBS), including adaptive hyperplasia and absorption of glucose and amino acids.
METHODS: Forty Sprague-Dawley (SD) male rats with more than 85% small intestine resected were equally divided into five groups randomly: H-GH group (high dose at 7.5 IU/kg per day), M-GH group (moderate dose at 3.75 IU/kg per day), L-GH group (low dose at 1.88 IU/kg per day), SBS group and sham operation group. From the second to the 15th day after operation, all the GH-managed groups were treated by sc injection twice a day, while SBS group and sham group were managed with same volume normal saline for injection. All samples were gained by laparotomy under anesthesia at the 16th day after operation.
RESULTS: Weight loss of rats in H-GH group (36±4.4 g), which was the least among the four groups except sham group, was significantly less than that in SBS group (94±10.0 g) (P < 0.05). But preoperative body weight of rats in the four groups except sham group was not retrieved. Among all groups there was no significant difference in the length of jejunum and ileum, as well as no significant difference in the morphological variables of colon. Mucosal height of jejunum and ileum was greater in H-GH group and M-GH group (997±65.9 m, 752±79.3 m and 974±67.6 m, 788±75.1 m respectively) than those in SBS group (776±61.0 m, 664±64.0 m) (P < 0.05). Similarly, intestinal wall width of jejunum and ileum was also thicker in H-GH group and M-GH group (1142±65.4 m, 884±91.2 m and 1 145±78.7 m, 895±95.6 m respectively) than those in SBS group (848±194.7 m, 776±57.5 m) (P < 0.05). But mucosal height and intestinal wall width of jejunum and ileum in H-GH group were not significantly greater than those in M-GH group. Blood insulinlike growth factor 1 (IGF-1) concentration and PCNA index of liver did not differ among the five groups. No significant differences of blood glucose and amino acids concentrations were detected after nutritional administration among the five groups.
CONCLUSION: Treatment of SBS with GH only slows body weight decrease rather than promotes body weight gain by the support of enteral nutrition. GH enhances adaptive mucosal hyperplasia after massive resection of small intestine, while its enhanced effect does not parallel its dose increase. Because of GH resistance resulted from the SBS-induced malnutrition,elevation of blood IGF-1 is impaired and absorpton of glucose and amino acids is not enhanced.
- Citation: Su ZD, Qin HL. Effects of growth hormone on intestinal adaptation of rat with short bowel syndrome. Shijie Huaren Xiaohua Zazhi 2004; 12(3): 646-649
- URL: https://www.wjgnet.com/1009-3079/full/v12/i3/646.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i3.646
生长激素(GH)在短肠综合征(SBS)治疗中发挥着重要作用, 已发现GH能促进SBS残留肠道的适应性代偿和使营养素吸收增加. 但也有不少学者在动物实验或临床上未能发现GH显著促进肠道增生和吸收, 并认为GH的量效关系还有待明确. 我们应用肠内营养(EN)和GH治疗鼠SBS模型, 观察不同剂量GH对残留肠道代偿增生和吸收功能的影响.
♂SD鼠40只, 体质量约300 g. EN用能全力(荷兰Nutricia公司), GH为长春金赛药业产品.
术前禁食12 h, 不禁水. 用25 g/L戊巴比妥钠腹腔麻醉, 无菌条件下作上腹正中切口. SBS组和各剂量GH组保留距Treitz韧带约7 cm的空肠和距回盲部约7.5 cm的回肠, 切除85%以上的小肠, 两断端用7-0无创缝线间断吻合; 假手术组在距回盲部约7.5 cm处切断回肠后再用7-0无创缝线间断吻合, 模拟手术创伤. 术后鼠各置一笼分别饲养. 40只SD鼠随机分成假手术组(Sham)、SBS组、大剂量GH 组(H-GH组)、中剂量GH组(M-GH组)和小剂量GH组(L-GH组)5组, 每组8只. 均在术后当日给50 g/L GNS 150 mL喂服, 术后1 d给能全力75 mL加生理盐水(NS)75 mL喂服, 术后2-15 d给能全力125mL加NS125 mL喂服. 术后2-15 d 2次/d scGH, H-GH组7.5 IU/kg, M-GH组3.75 IU/kg, L-GH组1.88 IU/kg, 假手术组和SBS组注射等量生理盐水. 手术当日和术后4 d、8 d、12 d、16 d称体质量. 术后16 d晨经一夜禁食后再次麻醉开腹. 分别测量残留空肠和回肠的长度, 并作形态学检查, 测量肠黏膜厚度和肠壁全厚等, 免疫组化法测定肝PCNA表达情况, 相应图像处理软件采用HPIAS-1000高清晰度彩色病理图文报告管理系统(同济大学出版). 营养吸收检测参考Kato et al (J Pedia Surg 1998; 33: 235-239)的方法, 距回盲部3 cm处选取一段长1.5 cm的回肠, 两端结扎, 两结扎处内侧分别用22GA静脉留置针插管, 近端为进管, 远端为出管, 开放. 等量能全力和NS混合置入10 mL注射器并接进管, 用Intima-Ⅱ型静脉推泵(日本BD公司)推注, 2 mL/h, 持续1.5 h. 血标本用22GA静脉留置针穿刺下腔静脉抽取, 能全力推注前抽血测血糖(Glu)和血氨基酸(AA) 浓度, 1.5 h后再测血Glu, AA(比色法)和IGF-1浓度(ELISA法).
统计学处理 采用mean±SD表示. 用SPSS11.0版统计软件包统计分析, 数据的方差齐性用Levene方法检验, 比较用One-Way ANOVA 过程, 若存在显著差异, 再用SNK方法两两比较, P<0.05表示统计学上有显著差异.
所有实验动物无伤口裂开、肠瘘、腹腔感染等并发症发生, 无死亡.
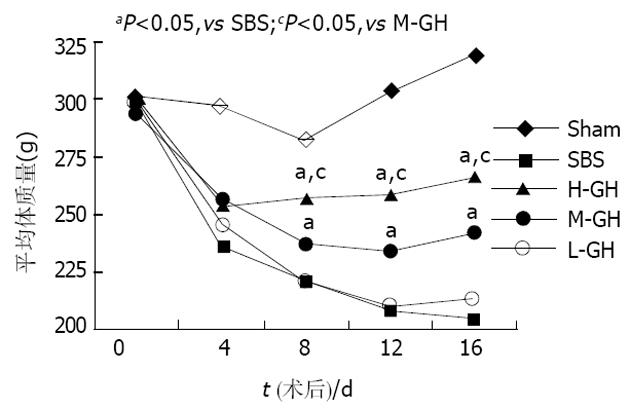
术后所有SBS动物体质量均下降. 其中SBS组和L-GH组下降最明显, H-GH组下降幅度最小. 比较术后4, 8, 12, 16 d体质量, 各组间存在显著差异(P = 0.000). H-GH组和M-GH组均显著高于SBS组(P<0.05), 且H-GH组显著高于M-GH组(P<0.05)(图1).
各组空、回肠长度无显著差异. H-GH组 和M-GH组空、回肠黏膜厚度均显著大于SBS组(P<0.05), 而SBS组又显著大于假手术组(P<0.05), 但SBS 组和L-GH组间无显著差异. 各组结肠组织学指标差异不显著(表1).
| 分组 | 空肠(cm) | 回肠(cm) | 空肠(μm) | 回肠(μm) | 结肠(μm) | |||||
| d1 | d16 | d1 | d16 | 黏膜厚度 | 肠壁厚度 | 黏膜厚度 | 肠壁厚度 | 黏膜厚度 | 肠壁厚度 | |
| sham | 576±61 | 716±68 | 531±48 | 652±66 | 611±109 | 788±112 | ||||
| SBS | 6.9±0.6 | 10.0±0.6 | 7.2±1.0 | 8.0±1.3 | 776±61a | 848±195 | 664±64a | 776±57a | 711±206 | 888±182 |
| H-GH | 7.0±0.0 | 10.6±1.3 | 7.0±0.0 | 8.6±1.3 | 997±66b | 1 142±65b | 752±79b | 884±91b | 554±103 | 767±126 |
| M-GH | 7.1±0.2 | 10.4±1.1 | 7.9±1.0 | 8.8±1.2 | 974±68b | 1 145±79b | 788±75b | 895±96b | 632±152 | 649±76 |
| L-GH | 7.3±0.3 | 9.9±0.8 | 7.8±0.3 | 8.4±0.4 | 773±144a | 949±174 | 694±33a | 809±42a | 832±179 | 810±60 |
比较各组能全力推注前血AA浓度及其推注前后浓度差, SBS组与其余各组间存在显著差异(P分别为0.020和0.001), 能全力推注后血AA浓度各组差异不显著. 相关血Glu指标、血IGF-1浓度各组间无显著差异. 各组肝PCNA无显著差异(表2).
| 分组 | AA (mmol/L) | Glu (mmol/L) | IGF-1 (μg/L) | 肝PCNA/% | ||||
| AA1 | AA2 | AA2-1 | Glu1 | Glu2 | Glu2-1 | |||
| sham | 4.52±0.41 | 5.85±0.76 | 1.33±0.47 | 5.50±2.50 | 10.21±1.46 | 4.71±1.17 | 22.56±20.91 | 0.38±0.22 |
| SBS | 4.03±0.39a | 7.83±1.91 | 3.80±0.70a | 7.90±0.55 | 13.50±6.07 | 5.60±5.93 | 13.38±1.73 | 0.65±0.32 |
| H-GH | 4.74±1.00 | 6.44±0.77 | 1.70±0.36 | 11.18±5.69 | 18.04±5.74 | 6.86±1.16 | 20.79±3.32 | 0.28±0.18 |
| M-GH | 5.57±0.80 | 6.88±0.15 | 1.32±0.81 | 9.75±1.09 | 15.47±3.42 | 5.72±0.81 | 16.39±5.40 | 0.33±0.20 |
| L-GH | 5.21±0.51 | 6.06±0.34 | 0.85±0.28 | 7.93±2.15 | 13.11±2.04 | 5.17±0.48 | 13.35±7.78 | 0.45±0.36 |
已知GH能促进蛋白质合成, 抑止葡萄糖氧化, 增加糖原储存, 且小肠黏膜有GH受体[1], 理论上存在着促进体质量增加的可能. 有研究报告SBS实验动物用GH治疗后体质量显著大于未用GH治疗的动物[2-3], 说明GH治疗对SBS发生后的体质量变化存在着影响, 而另一些动物实验和临床试验未发现GH对体质量增加有影响[4-7]. 本结果提示, GH对体质量的这种影响就是GH延缓了体质量下降的程度, 并且这种延缓程度与GH剂量有关, 随着GH剂量的增加, 体质量下降程度则呈相应减缓的态势. 有关GH是否促进SBS残留小肠代偿性增生, 多数学者持肯定意见[8-12], 认为GH能促进小肠黏膜显著增生. 本实验结果显示SBS组空、回肠黏膜厚度均显著大于假手术组, 而H-GH组和M-GH组又显著大于SBS组, 说明SBS的发生诱导了小肠黏膜代偿增生, 而GH能显著加强这种代偿增生. 同时也发现SBS组空、回肠黏膜厚度与L-GH组差异不显著, 表明GH剂量和小肠黏膜增生间的量效关系并不同步, 即GH在一定剂量以下, 促进残留小肠增生的作用不明显, 而超过一定剂量, GH才能显著促进增生, 但这种促进作用并不随GH剂量进一步增加而加强. 首先, 这可能与SBS引起的严重营养不良导致GH受体功能障碍有关, 被称之为对GH的抵抗状态, 只有当GH剂量大到足以克服这种GH抵抗状态, 才能促进小肠黏膜增生. 其次, 当GH剂量继续增加时, 所有小肠黏膜柱状细胞的绒毛和隐窝上的GH受体都被结合, 即使增加GH的剂量也无法获得更强的刺激增生作用. 我们既往研究表明用很大剂量GH(每日15 IU/kg)也不能进一步促进代偿增生[13]. GH促进残留小肠代偿增生的作用还与营养供给方式有关[14-15]. EN能增强GH的这种促进作用, 使较小剂量GH就能有效促进残留小肠的代偿增生. 在其他条件基本相同的情况下, Eizaguirre et al[8]给SBS鼠全胃肠外营养(TPN)支持, 每日用GH1 mg/kg才使残留空肠黏膜显著增生; Zhou et al[9]给EN, 每日用GH 0.45 mg/kg就使空肠黏膜显著增生. 而且, 在SBS较重的情况下, EN可使GH剂量不显著增加而仍达到原有的促进代偿增生的效果. 本实验与Eizaguirre et al[8]的实验在鼠术前体质量和GH用量及疗程等方面基本相同, 我们给予EN, Eizaguirre et al给TPN, 尽管我们切除的小肠长度要大于Eizaguirre et al做的(85% vs 80%), 但空肠黏膜的代偿增生比例却是相似的(29% vs 28%). 目前已认识到SBS发生后给予所谓肠道休息的治疗是无益的, 因为他使肠道处于较长时间的废用状态, 使残留肠道黏膜得不到食物刺激和食物中有关营养素的直接营养以及肝胰等分泌的消化液的经常作用等. 早期EN有利于残留肠道的代偿增生和消化功能的尽快恢复, 这已被许多临床研究所证实[16-20]. 所以, 在临床实践中, 如果条件允许, 应尽可能早期多用EN. 此外, 本研究还发现H-GH组能显著刺激空、回肠肠壁厚度增加, 而SBS组、L-GH组只能使回肠肠壁厚度显著增加, 提示回肠对GH的刺激更敏感, 这与其他研究报告相一致[21]. 在临床治疗中首先应尽可能保留回肠, 另外考虑到大剂量GH时空肠也有代偿功能, 所以空肠也要注意保留.
SBS发生后尽管残留小肠代偿性增生, 但总吸收面积仍比原来小得多, 因而不能在短时间内吸收大量葡萄糖形成更高的血糖高峰. Sigalet et al[22]发现小肠黏膜对葡萄糖的转运速率在GH组和假手术组无显著差异, 而Tavakkolizadeh et al[23]报告GH未使小肠黏膜葡萄糖载体对葡萄糖的亲和力显著提高. 同样, GH也未使小肠黏膜上有关氨基酸转运系统的转运能力提高[24-25]. 本研究在能全力推注后测血糖、血氨基酸, 发现各组血糖、血氨基酸浓度无显著差异, 这与其他学者的结果是一致的[4-5,26], 提示随着GH剂量增大并未促进营养素的吸收. 已知, GH可通过IGF-1介导促代偿作用, 并可刺激肝脏合成IGF-1. Fadrique et al[27]报告GH组和SBS组血IGF-1浓度无显著差异, 本实验中也发现各组血IGF-1浓度无显著差异. 这些现象的发生被认为与机体营养状况有关[28]. 在营养不良情况下, 尤其是蛋白质缺乏时, GH升高IGF-1的作用减弱. 肝PCNA的变化可反映肝蛋白合成的变化情况, PCNA直接参与DNA前导链的合成, 与基因的复制、表达密切相关. 本实验各组肝PCNA表达无显著差异, 说明SBS引起肝PCNA的低表达, 并提示存在着IGF-1的合成障碍. 因此, 我们认为在GH治疗中, 营养状况是影响血IGF-1浓度的关键, 严重营养不良时即使加大GH剂量, 对IGF-1合成也无促进作用.
总之, 在SBS治疗中GH可减慢体质量下降幅度; 能促进残留小肠的代偿性增生, 但并非剂量越大, 促代偿作用就越强; 对糖和氨基酸吸收基本无促进作用. 由于EN能直接刺激和营养残留小肠黏膜, 有利于后者的代偿增生, 因而在GH治疗中联用EN能有效地增强GH的疗效. 此外, 为了尽快改善SBS患者的全身营养状况, 综合治疗中也有必要在GH治疗的同时, 视情况结合使用PN和谷氨酰胺等治疗以达到更为理想的效果[29-33].
编辑: N/A
| 1. | Shulman DI. Gastrointestinal effects of growth hormone. Endocrine. 2000;12:147-152. [PubMed] [DOI] |
| 3. | Ljungmann K, Grofte T, Kissmeyer-Nielsen P, Flyvbjerg A, Vilstrup H, Tygstrup N, Laurberg S. GH decreases hepatic amino acid degradation after small bowel resection in rats without enhancing bowel adaptation. Am J Physiol Gastrointest Liver Physiol. 2000;279:G700-G706. [PubMed] |
| 4. | Szkudlarek J, Jeppesen PB, Mortensen PB. Effect of high dose growth hormone with glutamine and no change in diet on intestinal absorption in short bowel patients: a randomised, double blind, crossover, placebo controlled study. Gut. 2000;47:199-205. [PubMed] [DOI] |
| 5. | Jeppesen PB, Szkudlarek J, Høy CE, Mortensen PB. Effect of high-dose growth hormone and glutamine on body composition, urine creatinine excretion, fatty acid absorption, and essential fatty acids status in short bowel patients: a randomized, double-blind, crossover, placebo-controlled study. Scand J Gastroenterol. 2001;36:48-54. [PubMed] [DOI] |
| 6. | Garcia-Sancho Tellez L Jr, Gómez de Segura IA, Vazquez I, De Miguel E, Garcia-Sancho L. Growth hormone effects in intestinal adaptation after massive bowel resection in the suckling rat. J Pediatr Gastroenterol Nutr. 2001;33:477-482. [PubMed] [DOI] |
| 7. | Li-Ling , Irving M. The effectiveness of growth hormone, glutamine and a low-fat diet containing high-carbohydrate on the enhancement of the function of remnant intestine among patients with short bowel syndrome: a review of published trials. Clin Nutr. 2001;20:199-204. [PubMed] [DOI] |
| 8. | Eizaguirre I, Aldazabal P, Barrena MJ, Garcia-Arenzana JM, Ariz C, Candelas S, Tovar JA. Effect of growth hormone, epidermal growth factor, and insulin on bacterial translocation in experimental short bowel syndrome. J Pediatr Surg. 2000;35:692-695. [PubMed] [DOI] |
| 9. | Zhou X, Li YX, Li N, Li JS. Glutamine enhances the gut-trophic effect of growth hormone in rat after massive small bowel resection. J Surg Res. 2001;99:47-52. [PubMed] [DOI] |
| 10. | Zhou X, Li N, Li JS. Growth hormone stimulates remnant small bowel epithelial cell proliferation. World J Gastroenterol. 2000;6:909-913. [PubMed] [DOI] |
| 11. | Gu Y, Wu ZH. The anabolic effects of recombinant human growth hormone and glutamine on parenterally fed, short bowel rats. World J Gastroenterol. 2002;8:752-757. [PubMed] [DOI] |
| 12. | Scolapio JS. Treatment of short-bowel syndrome. Curr Opin Clin Nutr Metab Care. 2001;4:557-560. [PubMed] [DOI] |
| 14. | Ukleja A, Scolapio JS, Buchman AL. Nutritional management of short bowel syndrome. Semin Gastrointest Dis. 2002;13:161-168. [PubMed] |
| 15. | Jeppesen PB, Mortensen PB. Enhancing bowel adaptation in short bowel syndrome. Curr Gastroenterol Rep. 2002;4:338-347. [PubMed] [DOI] |
| 16. | Prasad TR, Bajpai M. Intestinal atresia. Indian J Pediatr. 2000;67:671-678. [PubMed] [DOI] |
| 17. | Kocoshis S. Small Intestinal Failure in Children. Curr Treat Options Gastroenterol. 2001;4:423-432. [PubMed] [DOI] |
| 18. | Li N, Zhu W, Guo F, Ren J, Li Y, Wang X, Li J. Rehabilitative therapy of short bowel syndrome: experimental study and clinical trial. Zhonghua Wai Ke Za Zhi. 2000;38:565-569. [PubMed] |
| 19. | Wilmore DW. Indications for specific therapy in the rehabilitation of patients with the short-bowel syndrome. Best Pract Res Clin Gastroenterol. 2003;17:895-906. [PubMed] [DOI] |
| 20. | Jeppesen PB, Mortensen PB. Experimental approaches: dietary and hormone therapy. Best Pract Res Clin Gastroenterol. 2003;17:1041-1054. [PubMed] [DOI] |
| 21. | Durant M, Gargosky S, Dahlstrom K, Fang R, Hellman B Jr, Castillo R. The role of growth hormone in adaptation to massive small intestinal resection in rats. Pediatr Res. 2001;49:189-196. [PubMed] [DOI] |
| 22. | Sigalet DL, Martin GR. Hormonal therapy for short bowel syndrome. J Pediatr Surg. 2000;35:360-3; discussion 364. [PubMed] [DOI] |
| 23. | Tavakkolizadeh A, Shen R, Jasleen J, Soybel DI, Jacobs DO, Zinner MJ, Ashley SW, Whang EE. Effect of growth hormone on intestinal Na+/glucose cotransporter activity. JPEN J Parenter Enteral Nutr. 2001;25:18-22. [PubMed] [DOI] |
| 24. | Ray EC, Avissar NE, Vukcevic D, Toia L, Ryan CK, Berlanga-Acosta J, Sax HC. Growth hormone and epidermal growth factor together enhance amino acid transport systems B0,+ and A in remnant small intestine after massive enterectomy. J Surg Res. 2003;115:164-170. [PubMed] [DOI] |
| 25. | Ray EC, Avissar NE, Vukcevic D, Toia L, Ryan CK, Berlanga-Acosta J, Sax HC. Growth hormone and epidermal growth factor together enhance amino acid transport systems B(0,+) and A in remnant small intestine after massive enterectomy. J Surg Res. 2003;113:257-263. [PubMed] [DOI] |
| 26. | Scolapio JS. Effect of growth hormone and glutamine on the short bowel: five years later. Gut. 2000;47:164. [PubMed] [DOI] |
| 27. | Fadrique B, Lopez JM, Bermudez R, Gomez de Segura IA, Vazquez I, De Miguel E. Growth hormone plus high protein diet promotes adaptation after massive bowel resection in aged rats. Exp Gerontol. 2001;36:1727-1737. [PubMed] [DOI] |
| 28. | Krsek M, Rosická M, Haluzík M, Svobodová J, Kotrlíková E, Justová V, Lacinová Z, Jarkovská Z. Plasma ghrelin levels in patients with short bowel syndrome. Endocr Res. 2002;28:27-33. [PubMed] [DOI] |
| 29. | Wu GH, Wu ZH, Wu ZG. Effects of bowel rehabilitation and combined trophic therapy on intestinal adaptation in short bowel patients. World J Gastroenterol. 2003;9:2601-2604. [PubMed] |
| 30. | Zhu W, Li N, Ren J, Gu J, Jiang J, Li J. Rehabilitation therapy for short bowel syndrome. Chin Med J (Engl). 2002;115:776-778. [PubMed] |
| 31. | Gu Y, Wu ZH, Xie JX, Jin DY, Zhuo HC. Effects of growth hormone (rhGH) and glutamine supplemented parenteral nutrition on intestinal adaptation in short bowel rats. Clin Nutr. 2001;20:159-166. [PubMed] [DOI] |
| 32. | Zhu W, Li N, Ren J. [Rehabilitation therapy for short bowel syndrome]. Zhonghua Yi Xue Za Zhi. 2001;81:868-870. [PubMed] |