修回日期: 2003-09-25
接受日期: 2003-11-13
在线出版日期: 2004-03-15
目的: 研究Cox-2在胃癌细胞中及胃黏膜组织中表达的意义, 探讨其表达与胃上皮细胞凋亡的关系.
方法: 将粗制H. pylori总蛋白与胃上皮细胞株MKN28, AGS共同孵育, 用RT-PCR及免疫组化染色法测定孵育前后细胞Cox-2表达的情况, 同时检测了40例患者胃镜活检胃黏膜病变标本的Cox-2蛋白的表达及H. pylori感染情况. 用流式细胞术观察H. pylori、选择性Cox-2抑制剂NS-398及二者共同诱导细胞凋亡的情况.
结果: 与H. pylori孵育后的MKN28细胞株中Cox-2表达增加; Cox-2蛋白在与H. pylori共同孵育前后的MKN28细胞株中表达强度分别为0.26和0.40(P<0.05), AGS细胞中为0.29和0.31(P>0.05); Cox-2在胃癌中的表达明显高于浅表性胃炎(CSG)、萎缩性胃炎(CAG)组(P<0.05). 流式显示, 与H. pylori孵育24, 48 h MKN28细胞凋亡率分别为1.0%和5.7% (P<0.01); 与10, 100, 200 mol/L NS-398孵育24 h及48 h的MKN28细胞凋亡率分别为1.2%, 14.0%, 27.5%及1.5%, 31.2%, 51.8%, 具有浓度、时间依赖性(P<0.01). 与H. pylori, NS-398共同孵育24, 48h的MKN28细胞凋亡率分别为12.2%, 25.0%, 其凋亡率低于单用NS-398 (P<0.01).
结论: H. pylori感染上调MKN28细胞中Cox-2的表达; Cox-2基因可抑制细胞凋亡, 在胃癌发生发展过程中起重要作用, 可能为胃癌形成的机制之一.
引文著录: 余琴, 刘南植, 龚建平. 幽门螺杆菌对胃上皮细胞Cox-2表达与凋亡的影响. 世界华人消化杂志 2004; 12(3): 630-634
Revised: September 25, 2003
Accepted: November 13, 2003
Published online: March 15, 2004
AIM: To investigate the expression of Cox-2 in MKN28 and AGS gastric cells and gastric mucosal lesions with H. pylori infection, to determine whether Cox-2 gene expression by H. pylori infection could influence gastric cell apoptosis and to identify the relationship between Cox-2 and gastric carcinoma.
METHODS: The total H. pylori proteins of various concentrations were incubated with MKN28 and AGS gastric cells in vitro. RT-PCR and S-P immunohistochemical staining were used to detect the expression of Cox-2 before and after the incubation. 40 patients who underwent endoscopy were detected with S-P method. Apoptosis induced by H. pylori or selective Cox-2 inhibitor NS-398 or both was characterized by cell cycle kinetics with flow cytometry.
RESULTS: Expression of Cox-2 in MKN28 gastric mucosal cells incubated with H. pylori was significantly higher than that in non-incubated cells. Expression of Cox-2 protein in MKN28 gastric cells before and after incubated with H. pylori was 0.26 and 0.40 respectively (P<0.05), but in AGS gastric cells, the expression of Cox-2 protein was 0.29 and 0.31 before and after the incubation (P>0.05). Expression of Cox-2 protein in gastric carcinoma (GC) was higher than that in chronic superficial gastritis (CSG) and chronic atrophic gastritis (CAG) (P<0.05). The apoptosis rate when cells were incubated with H. pylori for 24h and 48h was 1.0% and 5.7% (P<0.05). Apoptofic cells were also observed after treated with 10, 100, 200 mol/L NS-398 for 24 h and 48 h, and apoptosis rate was 1.2%, 14.0% and 27.5%, and 1.5%, 31.4% and 51.8% respectively. However, the apopotosis induced by H. pylori and NS-398 was lower than that induced by NS-398 alone (P<0.01).
CONCLUSION: H. pylori upregulates Cox-2 expression in MKN28 gastric mucosal cells in vitro. Cox-2 can inhibit the apoptosis, which may promote gastric carcingenesis.
- Citation: Yu Q, Liu NZ, Gong JP. Cyclooxygenase-2 expression in gastric mucosal cells with H. pylori infection and its relationship with apoptosis. Shijie Huaren Xiaohua Zazhi 2004; 12(3): 630-634
- URL: https://www.wjgnet.com/1009-3079/full/v12/i3/630.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i3.630
幽门螺杆菌(Helicobacter pylori, H. pylori)作为I类致癌因子, 已得到公认[1-4], 但尚无证据阐明H. pylori感染如何引起胃癌的发生. H. pylori感染可诱导Cox-2的表达[5-8], 并被认为是H. pylori感染增加胃癌发生危险性的可能机制之一. Cox-2过表达可引起局部产生较多的前列腺素(PGs), 同时他可作为分化和生长因子, 发挥类似免疫抑制剂及血管合成药物样作用, 促进肿瘤的增生发展[9-12]. 研究表明, NSAID药物的使用可以降低胃肠道肿瘤的发生[13-16], NSAID的作用靶点是Cox, 即花生四烯酸转化成前列腺素代谢中的限速酶, 其至少有两种亚型, Cox-1是一种看家基因, 其产生的前列腺素与胃肠道黏膜的完整性相关; Cox-2则是一种早期诱导基因, 其与炎症及肿瘤发生相关. 为此, 研究了H. pylori感染胃上皮细胞及胃黏膜病变Cox-2表达情况及其细胞生物学行为, 观察H. pylori, 不同浓度的选择性Cox-2抑制剂NS-398及二者共同诱导细胞凋亡的情况, 并对其凋亡的动力学及机制进行探讨.
人胃癌细胞MKN28(管状腺癌)由上海第二医科大学惠赠, AGS细胞(腺癌)购于中科院上海细胞生物研究所. 胃镜活检40例作胃黏膜组织学检查, 其中慢性浅表性胃炎(CSG)7例, 慢性萎缩性胃炎(CAG)7例, 肠化(IM)10例, 异型增生(Dys)6例, 胃癌(GC)10例, 其中低分化腺癌2例, 高分化腺癌2例, 黏液细胞癌4例, 印戒细胞癌2例.
人胃腺癌细胞系MKN28, AGS生长于RPMI1640培养液中, 按贴壁细胞传代培养法, 胰酶消化每2-3 d传代1次. 自胃溃疡患者活检胃黏膜组织中分离H. pylori, 37 ℃微需氧环境(50 mL/L O2, 100 mL/L CO2)培养. 将其超声粉碎(60 A×5 min ×3次, 间隔15 s), 20 000 r/min离心20 min, 取上清. 调整细胞浓度为1×109/L, 加入相当于1011CFu/L活菌量的H. pylori粗制总蛋白, 再加入RPMI1640 2 mL, 放入37 ℃孵育24 h, 以不加H. pylori的等体积的培养液为对照. RT-PCR: Cox-2上游引物序列: 5'-TCTGGTGCCTGGTCTGATGATGTA-3'; 下游引物序列为: 5'-CAGAAGGGGATGCCAGTGATAGAG-3'. 用Trizol法提取总RNA, 并进行逆转录. 待细胞与不同浓度的粗制H. pylori总蛋白 (相当于109-1011CFu/L的活菌量)孵育24 h后, 用SP法检测Cox-2蛋白的表达. 同法检测40例胃镜标本中Cox-2蛋白表达的情况. 调整细胞浓度为1×109/L, 加入相当于1011CFu/L活菌量的H. pylori, 不同浓度的NS-398(10, 100, 200 mol/L)及H. pylori +NS-398 (10, 100, 200 mol/L), 以不加H. pylori及NS-398的空白细胞为对照. 分别于培养24, 48 h后, PI染色(含Rnase 100 mg/L)上机测定凋亡细胞百分率及细胞同期.
统计学处理 所有实验数据以mean±SD表示. 采用t检验和精确概率法检验, 率的显著性差异检验选用方差分析, P<0.05认为差异有显著性.
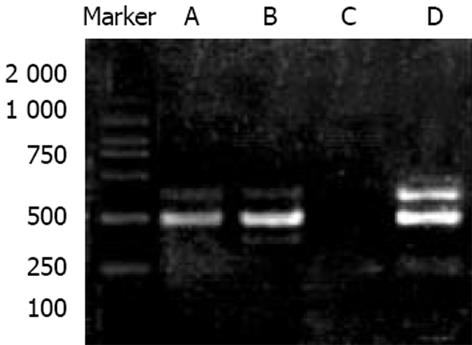
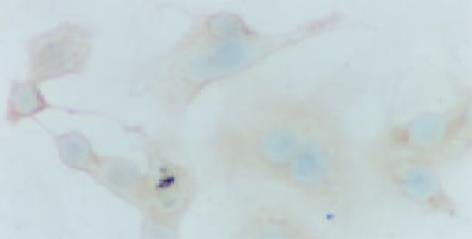
MKN28细胞的RT-PCR产物经电泳分析, 可见1条约314 bp的扩增带, 特异性好, 阴性对照无相应条带出现. 在与H. pylori总蛋白孵育前后的平均Cox-2/-actin为0.2698±0.0124和 0.6720±0.0206, 提示Cox-2mRNA在孵育后的细胞中表达水平增强(P<0.05, 图1). MKN28细胞分别与相当于1011, 1010, 109CFu/L的H. pylori粗制总蛋白孵育24 h的平均吸光度为0.40±0.13, 0.40±0.08和0.30±0.14; 未与H. pylori孵育的MKN28Cox-2表达的平均吸光度为0.26±0.18, 与1011, 1010CFu/L H. pylori孵育后Cox-2的表达相差显著(P<0.05)(图2, 3).
AGS细胞未与H. pylori孵育及与相当于1011, 1010, 109CFu/L的H. pylori粗制总蛋白孵育24 h的平均吸光度分别为0.29±0.22, 0.30±0.19, 0.32±0.24, 0.31±0.15 (P>0.05)(图4, 5).
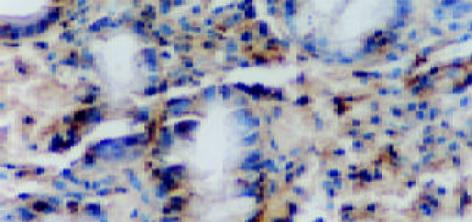
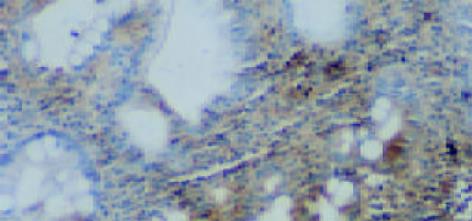
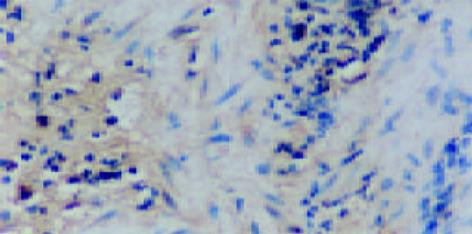
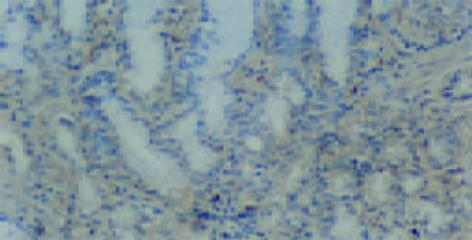
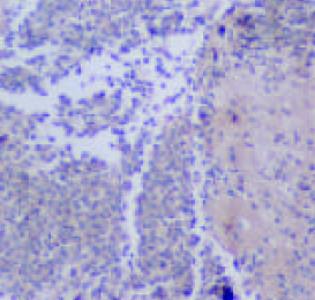
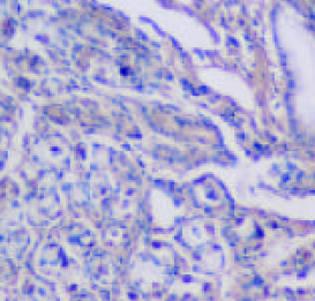
CSG, CAG, IM, Dys及GC中Cox-2表达呈平行递增趋势, GC与CSG, CAG中的表达差异有显著性(P<0.05, 表1). Cox-2主要表达在胃癌细胞中, 血管平滑肌细胞, 成纤维细胞, 炎性单核细胞及肠化上皮, 不典型增生腺上皮细胞亦表达, 胞质显色, 弥漫性分布; 胃炎, IM, Dys中H. pylori感染率与GC中H. pylori感染率差异有显著性(P<0.05)(图6-12).
| 病变 | n | Cox-2表达 | H. pylori感染n(%) |
| GC | 10 | 10 (100) | 0 (0.0) |
| Dys | 6 | 5 (83.3) | 4 (66.7) |
| IM | 10 | 8 (80.0) | 6 (60.0) |
| CAG | 7 | 4 (57.1) | 6 (85.7) |
| CSG | 7 | 2 (28.6) | 6 (85.7) |
H. pylori可诱导MKN28细胞的凋亡, 且具有时间依赖性(表2), NS-398亦可诱导MKN28细胞的凋亡, 具有时间、剂量依赖性(表3), H. pylori+NS-398对MKN28细胞凋亡, 也具有时间、剂量依赖性(表4), 但其凋亡率低于单纯NS-398组.
| 凋亡率 | G0/G1 | S | G2/M | |
| 对照 | 0.4±0.2 | 50.8±2.1 | 27.6±1.8 | 21.8±1.6 |
| 24 h | 1.0±0.3 | 58.7±2.5 | 19.3±1.6 | 21.5±0.9 |
| 48 h | 5.7±1.1b | 58.3±1.9 | 17.2±2.1 | 19.2±1.4 |
| NS-398 | 凋亡率 | G0/G1 | S | G2/M |
| 24 h | ||||
| 对照 | 0.5±0.3 | 51.1±2.6 | 29.5±1.6 | 19.9±2.8 |
| 10 mol/L | 1.2±0.8 | 48.1±3.0 | 28.1±2.1 | 23.0±1.9 |
| 100 mol/L | 14.0±2.1bd | 44.8±2.3 | 15.5±0.9 | 26.6±1.3 |
| 200 mol/L | 27.5±1.5bd | 34.9±0.8 | 5.8±2.2 | 32.4±2.3 |
| 48 h | ||||
| 对照 | 1.0±0.5 | 55.3±3.0 | 23.0±1.7 | 21.2±2.2 |
| 10 mol/L | 1.5±1.1 | 53.5±2.6 | 22.9±1.8 | 22.7±2.7 |
| 100 mol/L | 31.2±2.5b | 33.1±2.8 | 15.3±0.9 | 20.9±2.0 |
| 200 mol/L | 51.8±2.2b | 25.2±1.4 | 8.2±1.9 | 15.4±3.0 |
H. pylori感染与GC发生关系密切[17-23], 而Cox-2是黏膜炎症和上皮细胞生长的重要调节物, 该基因表达是对H. pylori感染直接的反应[5-6,24-27]. 因此, Cox-2的表达可能参与了H. pylori相关胃炎向癌前病变和胃癌的演变过程. 我们发现, H. pylori感染可上调MKN28细胞中Cox-2的表达, 且具有浓度依赖性, 当H. pylori小于或等于109CFu/L时, 对MKN28 Cox-2的表达并无明显影响; H. pylori对AGS细胞中Cox-2表达无明显影响, 这可能与不同胃上皮细胞中Cox-2启动子甲基化水平相关. Akhtar et al[28]研究了H. pylori感染的胃上皮细胞中Cox-2启动子甲基化对Cox-2表达及其活性的影响. MKN28细胞中的Cox-2启动子是未甲基化的, 而AGS细胞中Cox-2启动子是甲基化的. 用H. pylori刺激Cox-2启动子未甲基化的MKN28细胞, 其Cox-2表达明显增加, 而用H. pylori刺激Cox-2启动子甲基化的AGS细胞, 其Cox-2表达无明显增加. 但当用H. pylori感染经去甲基化药物处理后的AGS, Cox-2表达呈5-10倍地增加, 表明Cox-2启动子甲基化的缺失可能促进了Cox-2表达和H. pylori感染诱导胃癌的发生. 胃上皮细胞Cox-2启动子甲基化的缺失可能H. pylori感染诱发胃癌的一个中心事件, 可促进Cox-2表达导致细胞凋亡和增生的失衡[29-31].
本结果表明, 从CSG→CAG→IM→Dys→GC, Cox-2表达率增加, 可能为胃癌形成的早期事件. CSG, CAG, IM, Dys中H. pylori感染率与GC中H. pylori感染率差异有显著性, 这与胃癌所致胃内H. pylori生存环境改变有关. 如果Cox-2为胃肿瘤形成的早期事件, 则胃内炎症及H. pylori感染为胃癌的更早期事件. H. pylori感染致炎症, 产生大量的炎性因子激活Cox-2, 这可能为诱发癌变的机制之一. Cox-2除了使细胞凋亡、增生失衡外, 还可促进肿瘤细胞相关血管的生成, 增加癌细胞的侵袭性, 激活基质金属蛋白酶2降解细胞外基质, 产生促血小板凝集的血栓烷等, 从而有助于肿瘤的侵袭和转移[32]. 在体外, H. pylori粗制总蛋白可诱导细胞凋亡, 并且孵育时间越长, 细胞凋亡越多. 选择性Cox-2抑制剂NS-398能有效地促进MKN28细胞的凋亡, 且具有时间、浓度依赖性. 作用24 h时, 其凋亡率不很明显, 但细胞周期明显受阻, 停滞于G2期; 作用48 h时, 其凋亡率明显升高. 不同浓度NS-398对细胞凋亡影响亦不相同. 当用小剂量(10 mol/L)作用时, 细胞凋亡不明显, 细胞周期受阻也不明显, 提示10 mol/L NS-398并不能有效抑制Cox-2, 诱导细胞凋亡; 而100, 200 mmol/LNS-398使MKN28细胞凋亡明显增加. 我们还发现, 尽管H. pylori或NS-398单因素均可促进MKN28细胞的凋亡, 但二者同时作用于细胞时, 并未出现凋亡增加的情况, 相反, 其凋亡率竟远低于单纯用药组, 但其G2阻滞明显增加. 可能是由于H. pylori感染上调了细胞Cox-2的表达, 而Cox-2促进了细胞的增生, 抑制了凋亡, 所以加同浓度的Cox-2抑制剂时, 其凋亡少于仅加同剂量的用药组.
总之, 我们认为H. pylori感染增加胃癌发生的危险性, 可能与其上调Cox-2表达及其相关事件有关. 选择性Cox-2抑制剂的上市可望为胃癌的NSAID化学预防提供有利优势[33-34].
| 1. | Wang KX, Wang XF, Peng JL, Cui YB, Wang J, Li CP. School of medicine, Anhui university of science and technology, Huainan, Anhui Province, China. World J Gastroenterol. 2003;9:2501-2504. [PubMed] |
| 2. | Yang GB, Hu FL, Lu YY. Department of gastroenterology, first hospital, peking university, Beijing, China. Zhonghua Yixue Zazhi. 2003;83:1331-1335. [PubMed] |
| 3. | Li BQ, Zhang JZ, Zou QH, He LH, Yan XM. Department of diagnosis, Institute for communicable disease control and prevention, chinese center for disease control and prevention, Beijing, China. Zhonghua Liuxingbingxue Zazhi. 2003;24:439-442. [PubMed] |
| 4. | Bergin IL, Sheppard BJ, Fox JG. Division of comparative medicine, Massachusetts institute of technology, Cambridge, Massachusetts USA. Dig Dis Sci. 2003;48:475-485. [PubMed] [DOI] |
| 5. | Guo XL, Wang LE, Du SY, Fan CL, Li L, Wang P, Yuan Y. Cancer institute, the first hospital, china medical university, shenyang 110001, liaoning province, China. World J Gastroenterol. 2003;9:246-249. [PubMed] [DOI] |
| 6. | Guo X, Wang L, Yuan Y. Cancer institute, the first clinical college, china medical university, Shenyang, China. Zhonghua Yixue Zazhi. 2002;82:868-871. [PubMed] |
| 7. | Wambura C, Aoyama N, Shirasaka D, Sakai T, Ikemura T, Sakashita M, Maekawa S, Kuroda K, Inoue T, Ebara S. Second department of internal medicine and department of endoscopy, kobe university school of medicine, Japan. Helicobacter. 2002;7:129-138. [PubMed] [DOI] |
| 8. | Romano M, Ricci V, Memoli A, Tuccillo C, Di Popolo A, Sommi P, Acquaviva AM, Del Vecchio Blanco C, Bruni CB, Zarrilli R. Dipartimento di biologiae patologia cellularee molecolare "L. califano", universita federico ii, via pansini, napoli, Italy. J Biol Chem. 1998;273:28560-28563. [PubMed] [DOI] |
| 9. | Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Second department of surgery, tokyo medical and dental university, Tokyo, Japan. Cancer. 2001;91:1876-1881. [PubMed] [DOI] |
| 10. | Joo YE, Rew JS, Seo YH, Choi SK, Kim YJ, Park CS, Kim SJ. Department of internal medicine, chonnam national university medical school, gwangju, Korea. Clin Gastroenterol. 2003;37:28-33. [PubMed] [DOI] |
| 11. | Shi H, Xu JM, Hu NZ, Xie HJ. Department of gastroenterology, the first affiliated hospital, anhui medical university, anhui province, China. World J Gastroenterol. 2003;9:1421-1426. [PubMed] [DOI] |
| 12. | Yu HG, Li JY, Yang YN, Luo HS, Yu JP, Meier JJ, Schrader H, Bastian A, Schmidt WE, Schmitz F. Department of gastroenterology, renmin hospital of wuhan univeristy, jiefang road 238, China. Cancer Lett. 2003;195:43-51. [PubMed] [DOI] |
| 13. | Wang WH, Huang JQ, Zheng GF, Lam SK, Karlberg J, Wong BC. Department of gastroenterology, first hospital, peking university, beijing, China. J Natl Cancer Inst. 2003;95:1784-1791. [PubMed] [DOI] |
| 14. | Sorensen HT, Friis S, Norgard B, Mellemkjaer L, Blot WJ, McLaughlin JK, Ekbom A, Baron JA. Department of clinical epidemiology, aarhus university and aalborg hospital, denmark. Br J Cancer. 2003;88:1687-1692. [PubMed] [DOI] |
| 15. | Bosetti C, Gallus S, La Vecchia C. Istituto di ricerche farmacologiche "Mario negri", via eritrea 62, milan, Italy. Eur J Cancer Prev. 2002;11:535-542. [PubMed] |
| 16. | Akre K, Ekstrom AM, Signorello LB, Hansson LE, Nyren O. Department of medical epidemiology, karolinska institutet, Sweden. Br J Cancer. 2001;84:965-968. [PubMed] [DOI] |
| 17. | Huang JQ, Hunt RH. Department of medicine, division of gastroenterology, mcmaster university medical centre, hamilton, Canada. Can J Gastroenterol. 2003;17:18B-20B. [PubMed] [DOI] |
| 18. | Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, Pajdo R, Drozdowicz D, Stachura J, Karczewska E, Hahn EG. Department of medicine i, university of erlangen-nuremberg, erlangen, Germany. Eur J Gastroenterol Hepatol. 2003;15:745-754. [PubMed] |
| 19. | Akre K, Ekstrom AM, Signorello LB, Hansson LE, Nyren O. Department of medical epidemiology, karolinska institutet, Sweden. J Epidemiol. 2003;13:162-168. |
| 20. | Touati E, Michel V, Thiberge JM, Wuscher N, Huerre M, Labigne A. Unite de programmation moleculaire et de toxicologie genetique, institut pasteur, paris, France. Gastroenterology. 2003;124:1408-1419. [PubMed] [DOI] |
| 21. | Sasaki A, Kitadai Y, Ito M, Sumii M, Tanaka S, Yoshihara M, Haruma K, Chayama K. Dept. of medicine and molecular science, graduate school of biomedical sciences, hiroshima university, hiroshima, Japan. Scand J Gastroenterol. 2003;38:153-158. [PubMed] [DOI] |
| 22. | Chen X, Wang MW, You WD. Department of gastroenterology, general hospital of PLA, Beijing, 100853, P.R. China. Ai Zheng. 2003;22:244-247. [PubMed] |
| 23. | Takeuchi K, Ohno Y, Tsuzuki Y, Ando T, Sekihara M, Hara T, Kuwano H. Department of surgery, tone chuo hospital, gunma, Japan. J Clin Gastroenterol. 2003;36:321-324. [PubMed] [DOI] |
| 24. | Caputo R, Tuccillo C, Manzo BA, Zarrilli R, Tortora G, Blanco Cdel V, Ricci V, Ciardiello F, Romano M. Dipartimento di internistica clinica e sperimentalecattedra di gastroenterologia, seconda universita di napoli, Italy. Clin Cancer Res. 2003;9:2015-2021. [PubMed] |
| 25. | Seo JH, Kim H, Kim KH. Department of pharmacology and institute of gastroenter- ology, brain korea 21 project for medical science, yonsei university college of medicine, Korea. Ann N Y Acad Sci. 2002;973:477-480. [PubMed] [DOI] |
| 26. | Kim H, Lim JW, Kim KH. Dept. of pharmacology and institute of gastroentero- gy, yonsei university college of medicine, Korea. Scand J Gastroenterol. 2001;36:706-716. [PubMed] [DOI] |
| 27. | Xiao F, Furuta T, Takashima M, Shirai N, Hanai H. First department of medicine, hamamatsu university school of medicine, hamamatsu, Japan. Aliment Pharmacol Ther. 2001;15:875-886. [PubMed] [DOI] |
| 28. | Akhtar M, Cheng Y, Magno RM, Ashktorab H, Smoot DT, Meltzer SJ, Wilson KT. Department of medicine, university of maryland school of medicine and veterans affairs maryland health care system, USA. Cancer Res. 2001;61:2399-2403. [PubMed] |
| 29. | Yu J, Leung WK, Lee TL, Tse PC, To KF, Sung JJ. Department of medicine and therapeutics, prince of wales hospital, the chinese university of Hong Kong, China. Int J Oncol. 2003;22:1025-1031. [PubMed] |
| 30. | Kikuchi T, Itoh F, Toyota M, Suzuki H, Yamamoto H, Fujita M, Hosokawa M, Imai K. First department of internal medicine, sapporo medical university, Japan. Int J Cancer. 2002;97:272-277. [PubMed] [DOI] |
| 31. | Song SH, Jong HS, Choi HH, Inoue H, Tanabe T, Kim NK, Bang YJ. Cancer research institute, seoul national university college of medicine, Korea. Cancer Res. 2000;61:4628-4635. [PubMed] |
| 32. | Joo YE, Rew JS, Seo YH, Choi SK, Kim YJ, Park CS, Kim SJ. Department of internal medicine, chonnam national university medical school, Korea. J Clin Gastroenterol. 2003;37:28-33. [PubMed] [DOI] |
| 33. | Tang C, Wang C, Tang L. Department of Gastroenterology, West China Hospital, Sichuan University, China. Chin Med J (Engl). 2003;116:373-377. [PubMed] |
| 34. | Li JY, Wang XZ, Chen FL, Yu JP, Luo HS. Department of gastroenterology, affiliated union hospital, fujian medical university, China. World J Gastroenterol. 2003;9:915-920. [PubMed] [DOI] |