修回日期: 2003-12-01
接受日期: 2003-12-16
在线出版日期: 2004-03-15
目的: 研究leptin受体在人大肠癌细胞系及大肠腺癌组织中的表达, 探讨与肿瘤血管生成、细胞增生之间的关系.
方法: 免疫组织化学方法+图像分析检测leptin受体, CD34及Ki67的表达, 并计算肿瘤微血管密度及细胞增生指数.
结果: leptin受体在SW480, HT29细胞以及大肠癌组织均有表达, 大肠腺癌组织的平均染色吸光度比正常大肠组织高(0.153±0.011 vs 0.115±0.071, P<0.05), 与Ki67指数呈正相关(r = 0.388, P<0.05); 血管内皮细胞表达leptin受体的大肠腺癌, 其MVD较高(45.100±10.000 vs 37.400±10.200, P<0.05), 二者之间正相关(r = 0.569, P<0.05); 大肠腺癌MVD、Ki67指数比正常组织高(41.500±10.700 vs 31.300±11.100, P<0.01; 0.458±0.108 vs 0.312±0.097, P<0.01); leptin受体的表达与大肠腺癌临床病理参数之间未见相关.
结论: leptin与大肠腺癌上的leptin受体结合后, 在促进肿瘤细胞增生及肿瘤组织的血管增生方面具有一定的作用.
引文著录: 吴江, 邓长生, 陈立平. 大肠腺癌组织leptin受体的表达意义. 世界华人消化杂志 2004; 12(3): 585-589
Revised: December 1, 2003
Accepted: December 16, 2003
Published online: March 15, 2004
AIM: To investigation the expression of leptin receptor in colorectal cell lines and colorectal adenocarcinoma tissue, and the relationship between its expression, vascular proliferation and tumor cell proliferation.
METHODS: The expression of leptin receptor, CD34 and Ki67 proteins was detected by immunohistochemistry in HT29 and SW480 cells and colorectal adenocarcinoma tissues. Leptin receptor, the MVD and cell proliferation exponent were analyzed by image analysis software.
RESULTS: Leptin receptor was expressed in HT29 cells, SW480 cells and all colorectal adenocarcinoma tissue. The normal large intestine tissue also had leptin receptor, but the mean of their absorbance was lower than that of colorectal adenocarcinoma tissue (0.153±0.011 vs 0.115± 0.071, P < 0.05). The MVD and Ki67 exponent of colorectal adenocarcinoma tissue were both higher than those of normal controls (41.500±10.700 vs 31.300±11.100, P < 0.01; 0.458±0.108 vs 0.312±0.097, P < 0.01). There was a positive correlation between leptin receptor and Ki67 exponent (r = 0.388, P < 0.05). The MVD of colorectal adenocarcinoma tissue which leptin receptor expressed in blood vessel endothelial cells was higher than the negative ones (45.100±10.000 vs 37.400±10.200, P < 0.05). The correlation could not be found between leptin receptor and clinical pathologic parameters of colorectal adenocarcinoma.
CONCLUSION: Leptin may have some roles to accelerate tumor cell proliferation and vascular proliferation in colorectal adenocarcinoma tissue after it binds leptin receptor.
- Citation: Wu J, Deng CS, Chen LP. Expression and significance of leptin receptor in colorectal adenocarcinoma. Shijie Huaren Xiaohua Zazhi 2004; 12(3): 585-589
- URL: https://www.wjgnet.com/1009-3079/full/v12/i3/585.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i3.585
肥胖基因(Ob基因)的表达产物leptin蛋白(瘦素)具有抑制摄食、增加能量支出、调节代谢、激素分泌、生殖和免疫等多种功能. leptin必须与不同靶器官上的leptin受体(leptin receptor, 也称Ob-R)结合方能发挥其生物学功能. 我们用免疫组织化学方法检测leptin受体在大肠癌细胞系SW480, HT29, 大肠腺癌组织及正常大肠组织中的表达, 并用抗CD34抗体标记肿瘤组织微血管密度(MVD), 用抗Ki67抗体标记增生期细胞, 检测肿瘤组织细胞增生指数, 探讨leptin受体表达与微血管密度、细胞增生指数的关系, 及其与大肠癌临床病理参数之间的相关性.
大肠癌细胞系SW480, HT29由武汉大学生命科学学院提供, 取细胞悬液均匀涂于多聚赖氨酸处理的载玻片上, 干燥后浸入冷丙酮中固定5-10 min, 自然干燥, 放-20 ℃冰箱保存备用. 2001/2002武汉大学中南医院手术切除大肠腺癌标本30例, 男18例, 女12例, 年龄25-79 (平均46.9±18.7岁), 正常大肠组织20例, 男10例, 女10例, 年龄28-72(平均56.3±12.8岁), 为大肠手术切除之正常断端, 两组之间无关系. 所有组织标本用40 g/L甲醛固定, 常规脱水, 透明, 浸蜡, 包埋, 制备4 m厚连续切片, 黏附于涂有多聚赖氨酸的载玻片上, 60 ℃烤片备用. 以上切片均经HE染色复查, 明确病理诊断. 兔抗人leptin receptor多克隆抗体(浓缩液, 工作浓度为1: 50)购于武汉博士德生物工程有限公司; 鼠抗人CD34mAb (工作液), 鼠抗人Ki67mAb(工作液)和通用型SP免疫组化试剂盒, 均购于北京中山生物技术有限公司.
1.2.1 免疫组化染色: leptin受体, CD34和Ki67蛋白质表达检测均采用免疫组化SP法. 石蜡切片(4 m)常规脱蜡至水化; 浸泡于抗原修复液中, 放入YWY781B型微波炉, 一档加热10 min; 取出自然冷却至室温后PBS (0.01 mmol/L, pH7.4)洗涤; 加30 mL/L H2O2(试剂A)阻断内源性过氧化物酶活性, 37 ℃孵育10 min, PBS洗涤; 加封闭用正常羊血清(试剂B)减少非特异性染色; 无须洗涤, 直接滴加相应的第一抗体, 4 ℃冰箱孵育过夜, 取出后37 ℃再孵育60 min, PBS洗涤; 加生物素标记的二抗(试剂C), 37 ℃孵育10 min, PBS洗涤; 加辣根过氧化物酶标记的链酶卵白素(试剂D), 37 ℃孵育10 min, PBS洗涤; 滴加新鲜配置的DAB显色5-8 min, 自来水冲洗; 苏木精复染5 min, 自来水冲洗; 浸入盐酸酒精分化1 min, 自来水冲洗; 自然返蓝后, 脱水, 透明, 中性树胶封片. 以PBS代替一抗作为阴性对照, 阳性对照由试剂公司提供. SW480, HT29细胞涂片leptin受体表达检测无须脱蜡和微波抗原修复过程, 其余步骤同上. 用外接Polaroid数码相机的Olympus显微镜对玻片进行观察照相, 使用HPIAS-1000高清晰彩色病理图文分析系统进行图像分析.
1.2.2 leptin受体: 先在低倍镜(×400)下寻找肿瘤区域, 以细胞质出现棕黄色作为阳性细胞, 转高倍镜视野(×400)摄像, 由图像分析软件检测该视野肿瘤细胞染色吸光度(A值), 计算5个视野的平均吸光度作为最终表达结果. 同时在肿瘤间质内寻找阳性染色的血管内皮细胞, 按有无血管染色分组.
1.2.3 肿瘤微血管密度: 先在低倍镜(×40)下扫视肿瘤区域, 选择其中高密度区, 转高倍镜(×200)记数被抗CD34抗体染成棕色的血管数目, 结果以5个视野的均数表示.
1.2.4 肿瘤细胞增生指数: 以细胞核出现棕黄色作为阳性细胞, 在高倍镜(×400)下用图像分析软件检测Ki67细胞阳性率, 结果以5个视野的均数表示.
统计学处理 leptin受体染色吸光度、微血管密度(MVD)、细胞增生指数均以mean±SD表示, 所得数据用SPSS11.0统计学软件进行t检验和方差分析, 用Pearson相关分析leptin受体和MVD、细胞增生指数的关系. P<0.05有显著性差异.
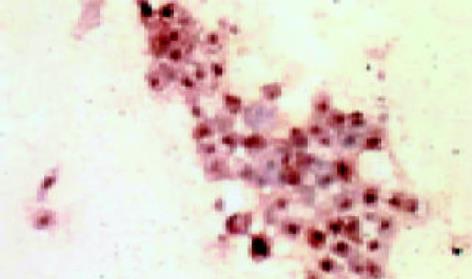
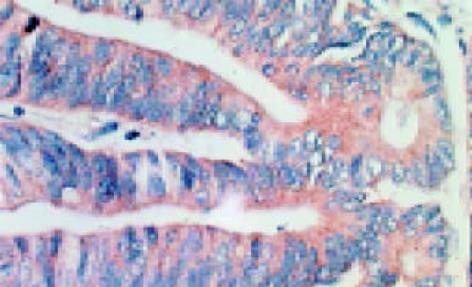
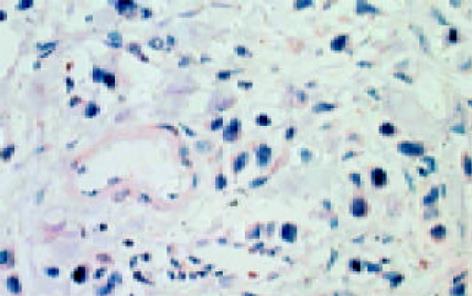
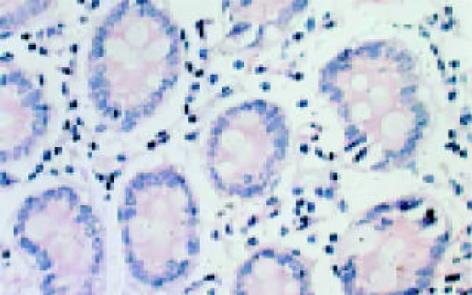
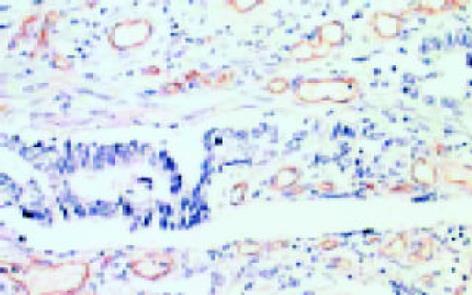
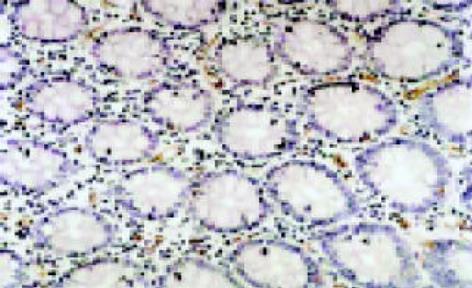
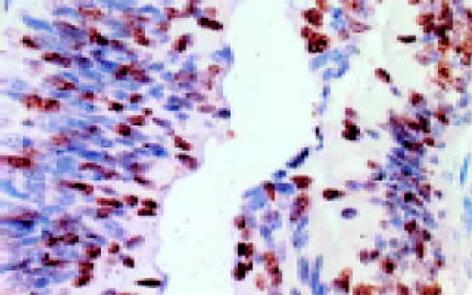
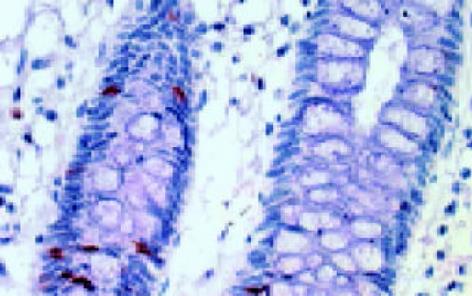
leptin受体在HT29 (图1)、SW480(图2)细胞及大肠腺癌组织细胞(图3)中均可见着色(30/30, 100.0%), 弥漫性分布, 呈均一棕黄色定位于肿瘤细胞胞质中, 部分肿瘤组织间质血管内皮细胞(图4)也有着色(16/30, 53.3%), 部分正常对照组织腺体细胞(图5)也可见阳性表达(15/20, 75.0%). 30例大肠腺癌组织中, leptin受体染色吸光度0.135-0.186, 平均0.153±0.011. 20例正常组织leptin受体染色吸光度0.000-0.185, 平均0.115±0.071, 大肠腺癌组与正常组之间存在显著性差异(t =2.366, P<0.05). CD34染色定位于血管内皮细胞膜上, 呈棕黄色, 在间质和癌巢都有分布, 微血管形态表现为管状、线状和点状. 30例大肠腺癌MVD(图6) 23.000-66.000, 平均41.500±10.700, 20例正常组织MVD(图7) 15.000-59.000, 平均31.300±11.100, 两组之间有显著性差异(t =3.255, P<0.01). Ki67染色定位于肿瘤细胞核, 呈棕色, 30例大肠腺癌Ki67指数(图8)0.291-0.650, 平均0.458±0.108, 正常大肠黏膜基底层细胞也可见表达, 20例正常组织Ki67指数(图9) 0.074-0.427, 平均0.312±0.097, 两组之间存在显著性差异(t =4.997, P<0.01).
16例大肠腺癌组织血管内皮细胞有leptin受体表达(16/30, 53.3%), 其MVD较之无表达的肿瘤组织高(45.100±10.000 vs 37.400±10.200, t =2.087, P<0.05), 且其leptin受体吸光度与MVD之间正相关(r = 0.569, P<0.05); 30例大肠腺癌组织leptin受体染色吸光度与Ki67指数呈正相关(r = 0.388, P<0.05), MVD与Ki67指数之间也呈正相关(r = 0.363, P<0.05).
leptin受体的表达在大肠腺癌组织高、中、低分化间无显著性差异(F = 1.120, P<0.05). Dukes'A, B, C分期之间的leptin受体表达未见差异(F = 0.343, P<0.05), 无淋巴结转移组(A+B期)与有淋巴结转移组(C期)间的leptin受体表达亦无差异(F = 0.037, P<0.05). 高分化大肠腺癌的MVD较中、低分化癌少(F = 4.095, P<0.05), 余未见差异(表1).
| 病理参数 | n | leptin受体染色吸光度 | MVD | Ki67指数 |
| 高分化 | 17 | 0.155±0.012 | 37.500±9.200a | 0.469±0.116 |
| 中分化 | 8 | 0.152±0.009 | 49.400±11.600 | 0.414±0.098 |
| 低分化 | 5 | 0.147±0.006 | 42.200±7.700 | 0.495±0.088 |
| Dukes'A期 | 8 | 0.155±0.008 | 45.900±7.100 | 0.442±0.123 |
| Dukes'B期 | 6 | 0.150±0.009 | 41.300±14.200 | 0.467±0.100 |
| Dukes'C期 | 16 | 0.153±0.013 | 39.300±10.700 | 0.463±0.109 |
| 淋巴结转移有 | 16 | 0.153±0.013 | 39.300±10.700 | 0.463±0.109 |
| 淋巴结转移无 | 14 | 0.152±0.009 | 43.900±10.500 | 0.453±0.110 |
人类Ob基因位于7号染色体(7q31.3), leptin是其编码产物, 由白色脂肪组织分泌入血. 人类leptin受体基因(Ob-R基因)位于l号染色体(1p31), leptin受体属于Ⅰ型细胞因子受体超家族, 是一种一次跨膜蛋白, 有5种亚型[1]: Ob-Ra-e, Ob-Rb的胞内结构域最长, 称为长亚型, 其余几种为短亚型. Ob-Ra在短亚型中占绝大多数. 因此一般就以Ob-Ra作为短亚型. leptin受体在体内分布于多种组织器官, 如中枢神经、心脏、胃、肝脏、胰脏、肾脏、前列腺、睾丸、卵巢、子宫、胎盘、皮肤和肌肉[2-8]等. leptin作为一种蛋白质激素, 具有促进多种细胞增生与分化的作用, 包括胎盘细胞、单核细胞、血管内皮细胞、自然杀伤细胞(NK)、骨及软骨细胞、胃黏膜细胞[9-14]等. 肿瘤细胞具有无限增生性和去分化性, 而leptin促进细胞增生与分化的特性, 在肿瘤的发生发展过程中可能发挥一定的作用. 我们用免疫组织化学方法发现, 在大肠癌细胞系SW480, HT29, 大肠腺癌组织细胞, 以及正常大肠组织的腺细胞中均有leptin受体表达. 30例大肠腺癌leptin受体平均表达强度比20例正常组织高, 差异具有显著性. 这种体内和体外、正常和异常中的表达提示, leptin受体的表达可能是大肠腺癌发生发展过程中的一个普遍性事件, 同时也说明大肠腺癌的发生发展与内分泌因素有一定的关系.
本实验显示, leptin受体在部分大肠腺癌间质血管内皮细胞上表达. 血管内皮细胞上有leptin受体表达的肿瘤组织, 其MVD较之无表达的肿瘤组织高, 且leptin受体的表达与MVD正相关. 说明其在促进肿瘤组织血管生成方面可能有一定作用, 体外实验也证明, leptin能促进血管内皮细胞的增生[11]. 大肠腺癌组织中的MVD明显高于正常对照, 说明确实存在着血管生成的增加. 肿瘤组织的血管生成, 为肿瘤的生长提供氧、营养物质和远处转移通道, 是肿瘤恶性生物学行为不可缺少的一环.
我们发现, 大肠腺癌组织肿瘤细胞增生活跃, 明显强于正常组. 大肠腺癌细胞增生指数与leptin受体的表达存在正相关关系, 说明leptin与其受体结合后, 能够促进大肠腺癌细胞的增生, 该结论与Hardwick et al[15]的体外实验结果一致. 此外本实验还发现大肠腺癌MVD与Ki67指数间存在正相关性, 血液供应丰富的区域其肿瘤细胞增生也活跃, 反之增生活跃的肿瘤细胞也能产生某些促血管增生的物质, 二者相互作用形成恶性循环网络, 而leptin及其受体可能参与其中. 我们未发现leptin受体的表达与大肠腺癌分化程度、Dukes'分期以及淋巴结转移等临床病理参数之间有相关性.
总之, 我们通过对大肠腺癌中leptin受体表达的研究, 将leptin这种内分泌激素与肿瘤联系起来, 初步探讨其在大肠腺癌发生发展过程中所发挥的作用以及可能的机制. 提示作为一个潜在的靶点, 在肿瘤的综合治疗方案中, leptin及其受体也许能找到一席之地. 由于大肠癌在西方和我国皆常见[16-28], 因而有良好的研究前景.
| 1. | Tena-Sempere M, Pinilla L, Zhang FP, González LC, Huhtaniemi I, Casanueva FF, Dieguez C, Aguilar E. Developmental and hormonal regulation of leptin receptor (Ob-R) messenger ribonucleic acid expression in rat testis. Biol Reprod. 2001;64:634-643. [PubMed] [DOI] |
| 2. | Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjørbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121-2131. [PubMed] [DOI] |
| 3. | Challier J, Galtier M, Bintein T, Cortez A, Lepercq J, Hauguel-de Mouzon S. Placental leptin receptor isoforms in normal and pathological pregnancies. Placenta. 2003;24:92-99. [PubMed] [DOI] |
| 4. | Reimer MK, Ahrén B. Altered beta-cell distribution of pdx-1 and GLUT-2 after a short-term challenge with a high-fat diet in C57BL/6J mice. Diabetes. 2002;51 Suppl 1:S138-S143. [PubMed] [DOI] |
| 5. | Ryan NK, Woodhouse CM, Van der Hoek KH, Gilchrist RB, Armstrong DT, Norman RJ. Expression of leptin and its receptor in the murine ovary: possible role in the regulation of oocyte maturation. Biol Reprod. 2002;66:1548-1554. [PubMed] [DOI] |
| 6. | Stallmeyer B, Kämpfer H, Podda M, Kaufmann R, Pfeilschifter J, Frank S. A novel keratinocyte mitogen: regulation of leptin and its functional receptor in skin repair. J Invest Dermatol. 2001;117:98-105. [PubMed] [DOI] |
| 7. | Mix H, Widjaja A, Jandl O, Cornberg M, Kaul A, Göke M, Beil W, Kuske M, Brabant G, Manns MP. Expression of leptin and leptin receptor isoforms in the human stomach. Gut. 2000;47:481-486. [PubMed] [DOI] |
| 8. | El-Hefnawy T, Ioffe S, Dym M. Expression of the leptin receptor during germ cell development in the mouse testis. Endocrinology. 2000;141:2624-2630. [PubMed] [DOI] |
| 9. | Caüzac M, Czuba D, Girard J, Hauguel-de Mouzon S. Transduction of leptin growth signals in placental cells is independent of JAK-STAT activation. Placenta. 2003;24:378-384. [PubMed] [DOI] |
| 10. | Martín-Romero C, Sánchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam68. Cell Immunol. 2001;212:83-91. [PubMed] [DOI] |
| 11. | Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, Jang Y, Cho SY, Kim HS. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95-102. [PubMed] [DOI] |
| 12. | Zhao Y, Sun R, You L, Gao C, Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003;300:247-252. [PubMed] [DOI] |
| 13. | Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405-415. [PubMed] [DOI] |
| 14. | Schneider R, Bornstein SR, Chrousos GP, Boxberger S, Ehninger G, Breidert M. Leptin mediates a proliferative response in human gastric mucosa cells with functional receptor. Horm Metab Res. 2001;33:1-6. [PubMed] [DOI] |
| 15. | Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121:79-90. [PubMed] [DOI] |
| 16. | Cui L, Jin HY, Cheng HY, Yan YD, Meng RG, Yu DH. Genetic detection of Chinese hereditary nonpolyposis colorectal cancer. World J Gastroenterol. 2004;10:209-213. [PubMed] |
| 17. | Xie LQ, Yu JP, Luo HS. Expression of estrogen receptor beta in human colorectal cancer. World J Gastroenterol. 2004;10:214-217. [PubMed] |
| 18. | Ma QY, Williamson KE, Rowlands BJ. Ethylene diamine tetraacetic acid-induced colonic crypt cell proliferation in rats. World J Gastroenterol. 2004;10:218-222. [PubMed] |
| 19. | Hu JK, Zhou ZG, Chen ZX, Wang LL, Yu YY, Liu J, Zhang B, Li L, Shu Y, Chen JP. Comparative evaluation of immune response after laparoscopical and open total mesorectal excisions with anal sphincter preservation in patients with rectal cancer. World J Gastroenterol. 2003;9:2690-2694. [PubMed] [DOI] |
| 20. | Liu HX, Ding YQ, Li X, Yao KT. Investigation of Epstein-Barr virus in Chinese colorectal tumors. World J Gastroenterol. 2003;9:2464-2468. [PubMed] |
| 21. | Li W, Li CB. Lack of inhibitory effects of lactic acid bacteria on 1,2-dimethylhydrazine-induced colon tumors in rats. World J Gastroenterol. 2003;9:2469-2473. [PubMed] |
| 22. | Zhao P, Hu YC, Talbot IC. Expressing patterns of p16 and CDK4 correlated to prognosis in colorectal carcinoma. World J Gastroenterol. 2003;9:2202-2206. [PubMed] [DOI] |
| 23. | Nan KJ, Qin HX, Yang G. Prognostic factors in 165 elderly colorectal cancer patients. World J Gastroenterol. 2003;9:2207-2210. [PubMed] [DOI] |
| 24. | Li GQ, Li H, Zhang HF. Mad2 and p53 expression profiles in colorectal cancer and its clinical significance. World J Gastroenterol. 2003;9:1972-1975. [PubMed] [DOI] |
| 25. | Fang JY, Lu J, Chen YX, Yang L. Effects of DNA methylation on expression of tumor suppressor genes and proto-oncogene in human colon cancer cell lines. World J Gastroenterol. 2003;9:1976-1980. [PubMed] [DOI] |
| 26. | Jiang YA, Luo HS, Zhang YY, Fan LF, Jiang CQ, Chen WJ. Telomerase activity and cell apoptosis in colon cancer cell by human telomerase reverse transcriptase gene antisense oligodeoxynucleotide. World J Gastroenterol. 2003;9:1981-1984. [PubMed] [DOI] |
| 27. | Wu AW, Gu J, Ji JF, Li ZF, Xu GW. Role of COX-2 in carcinogenesis of colorectal cancer and its relationship with tumor biological characteristics and patients' prognosis. World J Gastroenterol. 2003;9:1990-1994. [PubMed] [DOI] |
| 28. | Li XN, Ding YQ, Liu GB. Transcriptional gene expression profiles of HGF/SF-met signaling pathway in colorectal carcinoma. World J Gastroenterol. 2003;9:1734-1738. [PubMed] [DOI] |