修回日期: 2003-11-05
接受日期: 2003-12-08
在线出版日期: 2004-03-15
目的: 研究丝裂霉素与舒林酸合用对人胃腺癌SGC7901细胞的生长抑制、诱导凋亡及凋亡相关基因bcl-2和COX-2基因表达的影响.
方法: SGC7901胃癌细胞被分为三个实验组, 舒林酸组、丝裂霉素组和舒林酸与丝裂霉素联合组.应用光镜、激光共焦显微镜、MTT法、流式细胞仪和免疫组化技术研究三组药物作用后, 胃癌细胞的形态学变化、生长抑制、诱导凋亡和对凋亡相关基因Bcl-2 和COX-2表达的影响.
结果: 药物作用于细胞后, 可看到较为典型的细胞凋亡形态学变化: 细胞核固缩, 染色质凝集, 核碎裂, 染色质片段化, 凋亡小体形成等. 药物干预24 h, 联合组和MMC组对胃癌SGC7901细胞诱导的凋亡率分别为12.0%和7.20%. 对细胞的增生抑制以联合组最强, MMC次之, 舒林酸最弱.经MMC作用24 h后, COX-2和Bcl-2蛋白表达增强, 而联合组出现COX-2蛋白表达减弱, Bcl-2蛋白亦未出现明显升高.
结论: 人胃癌SGC7901细胞体外实验中, MMC联合舒林酸使用, 可使增生抑制加强. MMC可能由于上调COX-2、Bcl-2蛋白, 减弱了自身对SGC7901胃癌细胞的诱导凋亡, MMC联合舒林酸可抑制COX-2、Bcl-2表达, 从而提高MMC的抗癌效果.
引文著录: 颉永乐, 马力. 丝裂霉素联合舒林酸对胃癌SGC7901细胞诱导凋亡的研究. 世界华人消化杂志 2004; 12(3): 542-545
Revised: November 5, 2003
Accepted: December 8, 2003
Published online: March 15, 2004
AIM: To investigate the effects of mitomycin (MMC) with sulindac on the cell viability, apoptotic induction and expression of apoptosis-related gene Bcl-2 and cyclooxygenase-2 (COX-2) in gastric adenocarcinoma cell SGC7901.
METHODS: Human gastric cancer SGC7901 cells were divided into three groups, sulindac, MMC and sulindac with MMC. After treatment with drugs, cell viability was examined by MTT assay. Flow cytometry was used for the cell cycle distribution and apoptotic rates. The morphology of the cells was observed under light microscope and interactive laser cytometer. The expression of COX-2, Bcl-2 was determined by the immunocytochemical method.
RESULTS: After exposure for 12 h to three kinds of drugs, gastric cancer cells SGC7901 presented some morphologic features of apoptosis, including cell shrinkage, nuclear condensation, DNA fragmentation, formation of apoptotic bodies. The effects of growth inhibition were more obvious in cotreatment group with MMC and sulindac than MMC group. The apoptotic rates in cotreated cells and MMC-treated cells at 24 h after treatment were 12.0% and 7.2%, respectively. After exposure for 24 h to MMC, upregulation of COX-2 and Bcl-2 protein expression was noted, meanwhile, in cotreatment group, the levels of COX-2 were downregulated but the expression of Bcl-2 gene was not changed significantly.
CONCLUSION: MMC-induced apoptosis is reduced by upregulating the expression of COX-2 and Bcl-2 genes. MMC combined with sulindac can suppress growth of gastric cancer cells through induction of apoptosis which may be mediated by downregulation of apoptosis-related Bcl-2 gene and COX-2 gene.
- Citation: Xie YL, Ma L. Apoptosis induced by mitomycin with sulindac on human gastric cancer cell SGC7901. Shijie Huaren Xiaohua Zazhi 2004; 12(3): 542-545
- URL: https://www.wjgnet.com/1009-3079/full/v12/i3/542.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i3.542
COX-2基因表达的蛋白被认为是一种原癌蛋白(proonc-ogenic protein)[1], 在很多肿瘤组织中表达上调, 与肿瘤的发生与发展关系密切[2-12].非甾体类消炎药(nonstero-idal anti-inflammatory drugs, NSAID), 如阿司匹林、吲哚美辛、和舒林酸(sulindac)等, 这类药物可通过抑制环氧合酶-2 (cyclooxygenase-2 , COX-2)而起到抗肿瘤增生和诱导肿瘤细胞凋亡的作用[13-22]. NSAID中的选择性COX-2抑制剂赛来昔布(celecoxib)已被美国食品药品管理局(FDA)正式批准用于家族性腺瘤样息肉的防治[23]. 近年, NSAIDs抗胃癌细胞增生也屡见报道. 本实验旨在观察舒林酸和丝裂霉素(mitomycin, MMC)联合使用对体外培养胃腺癌SGC7901细胞增生的影响, 并通过检测COX-2和Bcl-2用药前后的变化, 探讨两种药物的合用是否可增强抗癌作用及可能的机制. 为临床治疗提供理论与实验依据.
人胃癌细胞株SGC7901 (中分化腺癌)购自中科院上海细胞生物研究所; RPMI1640培养基, 胰蛋白酶(Gibco公司); 四氮唑蓝(MTT)、碘化丙啶、舒林酸(sulindac)购自美国Sigma公司; 丝裂霉素(MMC)为日本协和株式会社产品; 山羊抗人COX-2多克隆抗体(Santa Cruz公司), Bcl-2单抗及SP试剂盒为福建迈新生物技术公司产品; 恒温CO2培养箱(美国Forma公司), 倒置显微镜(日本OLYMPUS公司), 酶联免疫检测仪(国营华东电子管长), 激光共焦显微镜(德国Leica SP2型), 流式细胞仪(EPICSXL型, 美国Coulter公司), 图像分析系统(四川联合大学).
将舒林酸和MMC以单药和两药联合的形式分为: 舒林酸组、MMC组和联合组. 实验时将溶解于培养液中的药物(舒林酸以DMSO预溶)加入培养细胞的新鲜培养液中, 使药物浓度达到: 舒林酸组, 含1 mmol/L (其中DMSO浓度不超过5 g/L); MMC组, 含1×10-3 g/L; 联合组, 含1 mmol/L的舒林酸和1×10-3 g/L的MMC.胃癌SGC7901细胞常规培养于含100 mL/L小牛血清、1×105u/L青霉素及1×10-3 g/L链霉素的RPMI1640培养液中, 置于37℃, 50 mL/L CO2培养箱内培养. 细胞为上皮样贴壁生长, 取对数生长期细胞用于实验. 将SGC7901细胞按每孔7×103个细胞接种于96孔板中, 24 h后换液, 加入药物, 每组设6个复孔, 并设含相同浓度DMSO的空白对照, 继续培养12 h, 24 h, 48 h, 每孔加入5 g/L的MTT 20 L; 作用4 h后, 弃去上清液, 每孔加DMSO溶液150 L, 振荡10 min, 使结晶物充分溶解, 在570 nm波长酶联免疫检测仪上测定各孔吸光度(A)值, 整个实验重复3次以上. 以时间为横轴, 吸光度(mean±SD)为纵轴绘制生长曲线.
1.2.1 形态学观察: 加入药物后, 在倒置显微镜下观察细胞变化形态; 分别于12 h, 24 h, 48 h取出处理后的单层附壁细胞爬片, 常规HE染色后观察; 将药物处理后的细胞制成细胞悬液, 取一滴细胞悬液与5 L吖啶橙溶液混匀点滴于清洁玻片上, 10 min后, 盖玻片封片. 激光共聚焦显微镜下观察、摄片.
1.2.2 流式细胞仪测定: 收集药物处理后的胃癌细胞, PBS洗涤后, 用4 ℃乙醇固定12 h以上, 离心除去乙醇, 加入10 g/L RNA酶溶液200 L, 37 ℃水浴15 min, 加入碘化丙啶, 上流式细胞仪检测凋亡百分率和各细胞周期所占百分比.
1.2.3 COX-2, Bcl-2蛋白检测: 细胞接种于盖玻片上, 分别处理, 作用24 h后取出玻片, PBS洗后, 950 mL/L乙醇固定30 min, 采用常规SP法, 细胞爬片依次用5 mL/L H2O2甲醇30 min, 羊血清室温封闭10 min, 滴加一抗后, 置4 ℃冰箱过夜, 再滴加生物素化二抗37 ℃孵育12 min, 滴加链亲和素-过氧化酶溶液37 ℃ 15 min, DAB显色、脱水、透明、封片. 实验中设置阳性对照, 阴性对照(以PBS代替一抗).
统计学处理 应用SPSS 8.0统计分析软件进行t检验和方差检验.
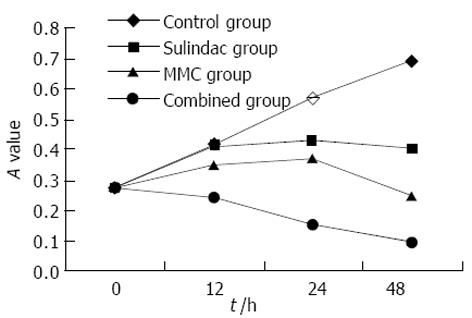
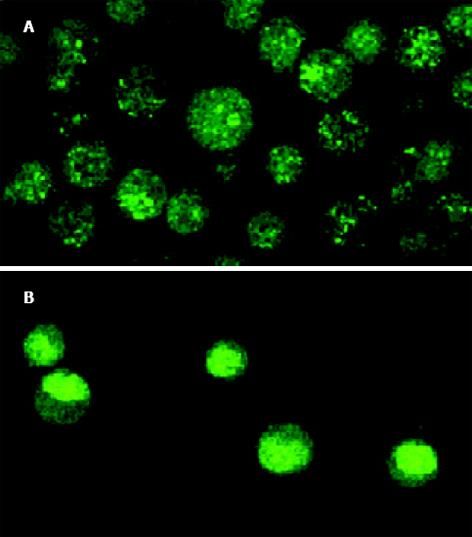
联合用药对细胞增生抑制强于丝裂霉素, 而舒林酸作用最弱(图1). 未用药的SGC7901细胞生长良好, 表现为梭形或多角形, 并可融合形成集落. 加药后, 出现伪足回缩, 细胞变圆、变小、部分细胞脱落漂浮于培养液中. 通过HE 染色, 可看到较为典型的细胞凋亡形态学变化: 细胞体积缩小, 核固缩深染, 部分裂解形成凋亡小体. 活细胞对荧光染料有拒染性, 故染色较浅, 激光共焦显微镜下观察, 可见正常细胞核形态饱满, 表现为弥散均匀的黄绿色荧光, 凋亡细胞则出现细胞核体积缩小, 荧光染色增强, 染色质呈致密浓染的块状或颗粒状荧光以及细胞膜出泡、凋亡小体形成. 上述表现以联合药物组最明显, 且凋亡细胞数量随着药物作用时间的延长而增加(图2).
药物作用24 h后, 流式细胞仪检测凋亡率和细胞周期分布, 可见细胞凋亡率明显增加, 且联合组细胞凋亡率明显高于丝裂霉素及其他组(P<0.01); 对细胞周期分布的影响, 即使G0/G1期细胞比例增高, 使S期细胞比例降低(表1).
对照组COX-2, Bcl-2蛋白均呈阳性表达. COX-2蛋白表达物质呈棕黄色颗粒状, 主要弥散分布于整个胞质或沿核膜周边呈线状分布, 强阳性时可反应于胞核; Bcl-2蛋白主要定位于胞膜、胞核及核膜. 采用图像分析系统检测免疫组化染色后细胞平均灰度值, 用此间接反映COX-2和Bcl-2蛋白表达量.舒林酸干预24 h后, COX-2、Bcl-2表达减弱, 而MMC则使两种蛋白表达增强, 合用后, COX-2表达减弱, Bcl-2与对照组比较无变化, 但较MMC组表达减弱(表2).
化疗耐药是肿瘤治疗中的难题, 研究耐药机制和寻求克服途径成为当今肿瘤治疗的重点[24-26]. 肿瘤细胞对化疗药物的耐药缘于多种机制, Bcl-2基因的异常表达可能与之有关[27]. 研究通过抑制Bcl-2的表达以增强化疗药物的敏感性, 如利用反义寡核苷酸封闭细胞凋亡抑制基因Bcl-2的表达, 可使细胞对顺铂、5-氟脲嘧啶的敏感性增强[28]. COX-2在许多肿瘤细胞中高表达, 如胃癌SGC7901细胞[29]. 有证据表明COX-2基因表达增强导致Bcl-2蛋白表达增加并引起细胞凋亡抑制[30], COX-2是Bcl-2的上游调节者, 通过作用于Bcl-2家族而影响肿瘤细胞凋亡的发生发展[31]. 最近研究者发现舒林酸在体外对人胃腺癌细胞株MKN45和MKN28有良好的增生抑制作用, 并能诱导2株细胞发生凋亡, 舒林酸诱导胃癌细胞凋亡与其抑制COX-2继而抑制Bcl-2蛋白有关[13]. 我们用MTT法证实MMC与舒林酸合用对SGC7901细胞的增生抑制强于MMC或舒林酸的单独干预; 流式细胞仪检测显示合用组较单药组更高的凋亡率; 药物干预24 h, MMC使COX-2, Bcl-2蛋白表达增多, 而合用后, COX-2, Bcl-2蛋白均较MMC组减少. 提示MMC可能通过增加COX-2, Bcl-2的表达减弱了自身了对胃癌细胞的凋亡诱导效应, 舒林酸抑制COX-2蛋白, 下调Bcl-2的表达, 从而增强了MMC的诱导凋亡. 国外学者报道[32], MMC作用于MKN74胃癌细胞后COX-2, Bcl-2上调, 而MMC与COX-2选择剂NS-398共同处理胃癌细胞后, 两种蛋白表达降低, 细胞凋亡有显著提高. 本实验中虽然未见到合用后Bcl-2较空白组减弱, 但较MMC作用后表达亦明显降低, 同时也可看到舒林酸对COX-2和Bcl-2蛋白的表达抑制作用.
细胞凋亡是一种主动的受基因调控的细胞自杀过程. 许多人体基因如Bcl-2, P53, C-ntyc及相关基因Bcl-2, bax, bad等参与凋亡的调控, 其中Bcl-2基因尤为重要, 他被看作是细胞凋亡调控的最后共同通路之一[33]. Bcl-2能抑制许多抗肿瘤药物的诱导细胞凋亡作用, 降低其细胞毒性[34]. 应用药物如COX-2抑制剂或反义基因治疗[2,35]与传统化疗药物协调使用, 可提高化疗效果, 减少药物剂量和药物副作用. 本实验观察到的结果提示舒林酸可能通过调节凋亡抑制基因Bcl-2, 从而使MMC产生化疗增效, 但COX-2与凋亡相关基因bax, Bcl-2之间的信号传递过程仍未完全清楚, 而且NSAID抗肿瘤作用机制中除了COX-2途径外, 还有COX-2非依赖途径[36-39], 包括对细胞周期的影响, 影响NF-κB的活性和P53的表达, RAS信号传递途径的调节等. 因此, NSAIDs抗胃癌机制中COX-2依赖和非依赖性途径可能均有参与, 但确切的作用机制及何种途径为主目前仍未明了. 我们的研究提示舒林酸和MMC在体外对胃癌SGC7901细胞的增生有一定的协同作用, 但在不同的细胞株及临床应用的有效性, 尚难确定, 须进行更为广泛、深入的研究.
编辑: N/A
| 1. | Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, Taketo MM, Evans JF. Chemoprention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res. 2001;61:1733-1740. [PubMed] |
| 2. | Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796-800. [PubMed] [DOI] |
| 3. | Davies G, Martin LA, Sacks N, Dowsett M. Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a possible role for COX-2 inhibitors in breast cancer chemoprevention. Ann Oncol. 2002;13:669-678. [PubMed] [DOI] |
| 4. | Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM. Cyclooxygenase 2 and prostaglandin E (2) receptor EP (2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res. 2002;62:506-511. [PubMed] |
| 5. | Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737-743. [PubMed] [DOI] |
| 6. | Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473-479. [PubMed] [DOI] |
| 7. | Ristimaki A, Nieminen O, Saukkonen K, Hotakainen K, Nordling S, Haglund C. Expression of cyclooxygenase-2 in human transitional cell carcinoma of the urinary bladder. Am J Pathol. 2001;158:849-853. [PubMed] [DOI] |
| 8. | Kulkarni S, Rader JS, Zhang F, Liapis H, Koki AT, Masferrer JL, Subbaramaiah K, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin Cancer Res. 2001;7:429-434. [PubMed] |
| 9. | Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol. 2001;76:26-30. [PubMed] [DOI] |
| 10. | Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, Kosuge T, Yoshida S. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001;91:333-338. [PubMed] [DOI] |
| 11. | Fantappie O, Masini E, Sardi I, Raimondi L, Bani D, Solazzo M, Vannacci A, Mazzanti R. The MDR phenotype is associated with the expression of COX-2 and iNOS in a human hepatocellular carcinoma cell line. Hepatology. 2002;35:843-852. [PubMed] [DOI] |
| 12. | Kakiuchi Y, Tsuji S, Tsujii M, Murata H, Kawai N, Yasumaru M, Kimura A, Komori M, Irie T, Miyoshi E. Cyclooxygenase-2 activity altered the cell-surface carbohydrate antigens on colon cancer cells and enhanced liver metastasis. Cancer Res. 2002;62:1567-1572. [PubMed] |
| 13. | Sun B, Wu YL, Zhang XJ, Wang SN, He HY, Qiao MM, Zhang YP, Zhong J. Effects of Sulindac on growth inhibition and apoptosis induction in human gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 2001;9:997-1002. [DOI] |
| 14. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. [PubMed] [DOI] |
| 15. | Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999;59:4574-4577. [PubMed] |
| 16. | Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of Nimesulid e on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483-487. [PubMed] [DOI] |
| 18. | Li JY, Yu JP, Luo HS, Yu BP, Huang JA. Effects of nonsteroidal anti-inflammatory drugs on the proliferation and cyclooxygenase activity of gastric cancer cell line SGC7901. Shijie Huaren Xiaohua Zazhi. 2002;10:262-265. [DOI] |
| 19. | Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, Krishnadath KK, Lutzke LS, Burgart LJ. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology. 2002;122:1101-1112. [PubMed] [DOI] |
| 20. | Mann M, Sheng H, Shao J, Williams CS, Pisacane PI, Sliwkowski MX, DuBois RN. Targeting cyclooxygenase 2 and HER-2/neu pathways inhibits colorectal carcinoma growth. Gastroenterology. 2001;120:1713-1719. [PubMed] [DOI] |
| 21. | Wight NJ, Gottesdiener K, Garlick NM, Atherton CT, Novak S, Gertz BJ, Calder NA, Cote J, Wong P, Dallob A. Rofecoxib, a COX-2 inhibitor, does not inhibit human gastric mucosal prostaglandin production. Gastroenterology. 2001;120:867-873. [PubMed] [DOI] |
| 22. | Zhang Z, DuBois RN. Par-4, a proapoptotic gene, is regulated by NSAIDs in human colon carcinoma cells. Gastroenterology. 2000;118:1012-1017. [PubMed] [DOI] |
| 25. | Wang SD, Zhang ZS, Zhang XQ, Zhou DY. The function of CPP32 in apoptosis of multidrug-resistance gastriccancer cells. Shijie Huaren Xiaohua Zazhi. 2001;9:1152-1156. [DOI] |
| 27. | Reed JC. Promise and problems of Bcl-2 antisense therapy. J Nat Can Inst. 1997;89:988-990. [DOI] |
| 29. | Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276-1280. [PubMed] |
| 30. | Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cells. 1995;83:493-501. [PubMed] [DOI] |
| 31. | Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245-4249. [PubMed] |
| 32. | Hsueh CT, Chiu CF, Kelsen DP, Schwartz GK. Selective inhibition of cyclooxygenase-2 enhances mitomycin-C-induced apoptosis. Cancer Chemother Pharmacol. 2000;45:389-396. [PubMed] [DOI] |
| 33. | Chen CJ, Sun YX, Zhou HG, Pan BR, Zheng SG, Hong XZ, Liu JH, Feng WY. Bcl-2 and p53 expressions in colorectal adenoma and carcinoma. Shijie Huaren Xiaohua Zazhi. 1998;6:683-685. [DOI] |
| 34. | Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151-157. [PubMed] |
| 35. | Wu GS, Wu XY, Zou SQ, Qiu FZ. Effects of cyclooxygenase-2 antisense vector on proliferation of human cholan-giocarcinoma cells. Shijie Huaren Xiaohua Zazhi. 2003;11:733-736. [PubMed] |
| 36. | Yamamoto H, Itoh F, Fukushima H, Hinoda Y, Imai K. Overexpression of cyclooxygenase-2 protein is less frequent in gastric cancers with microsatellite instablility. Int J Cancer. 1999;84:400-403. [PubMed] [DOI] |
| 37. | Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the NF-kappa B pathway. J Biol Chem. 1999;274:27307-27314. [PubMed] [DOI] |
| 38. | Gilhooly EM, Rose DP. The association between a mutated ras gene and cyclooxygenase-2 expression in human breast cancer cell lines. Int J Oncol. 1999;15:267-270. [PubMed] [DOI] |
| 39. | Majima M, Hayashi I, Muramatsu M, Katada J, Yamashina S, Katori M. Cyclo-oxygenase-2 enhances basic fibroblast growth factor-induced angiogenesis through induction of vascular endothelial growth factor in rat sponge implants. Br J Pharmacol. 2000;130:641-649. [PubMed] [DOI] |