修回日期: 2003-09-10
接受日期: 2003-09-24
在线出版日期: 2004-02-15
目的: 探讨抗纤软肝颗粒对PDGF诱导的肝星状细胞MEK-1和c-fos表达的影响.
方法: 采用无血清培养, 不同浓度的抗纤软肝颗粒温育肝星状细胞24 h后, PDGF-BB(10 pg/L)刺激24 h, 再加入上述浓度的抗纤软肝颗粒, 3 h后, 又加入PDGF-BB(10 pg/L)作用5 min, 然后收集细胞. 采用MTT法测定细胞增生, 免疫印迹化学发光法检测MEK-1, 原位杂交法检测c-fos mRNA.
结果: 无血清培养显示抗纤软肝颗粒对于PDGF诱导的细胞增生具有抑制作用, 并呈剂量依赖性, 抗纤软肝颗粒5 g/L、1.25 g/L 各组的MTT测定值分别为0.28±0.03和0.43±0.04, 与PDGF对照组(0.82±0.05)相比P<0.01; 对PDGF诱导的MEK-1及c-fos mRNA表达均有显著的抑制作用: 抗纤软肝颗粒5 g/L、1.25 g/L各组细胞的MEK-1表达水平分别为0.143±0.013、0.169±0.007, 与PDGF组(0.186±0.010)比较有显著性差异(P<0.01); c-fos mRNA表达水平分别为0.152±0.010、0.163±0.005, 与PDGF组(0.183±0.014)比较也显著减弱(P<0.01).
结论: 在所应用的剂量范围内, 抗纤软肝颗粒可抑制PDGF诱导的HSC增生, 其机制与干扰Ras-MEK-MAPK信号通路有关.
引文著录: 杨玲, 朱清静, 笪邦红, 张赤志. 中药抗纤软肝颗粒抑制PDGF诱导的肝星状细胞MEK-1和c-fos表达. 世界华人消化杂志 2004; 12(2): 347-350
Revised: September 10, 2003
Accepted: September 24, 2003
Published online: February 15, 2004
AIM: To investigate the effect of Kangxian ruangan keli (KXR) on the expression of MEK-1 and c-fos in hepatic stellate cell (HSC) indused by PDGF.
METHODS: In a serum-free culture system, HSC was treated with a KXR preparation for 24 hours, followed by stimulation with PDGF-BB for 24 hours. Then the cells were incubated again in the medium containing KXR for 3 hours stimulated with PDGF-BB for 5 minutes, and collected. The proliferation of HSC was examined using an MTT assay. MEK-1 was detected with Western blotting and visualized by the enhenced chemiluminescent (ECL) method. The expression of c-fos mRNA was analyzed with in situ hybridization.
RESULTS: The A values for the HSC growing in the media without and with addition of PDGF were 0.170±0.060 and 0.820±0.050, respectively. The PDGF-induced increase was hindered remarkably by KXR preparation in a dose-dependent manner. Reaction values for the systems with 5 g/L and 1.25 g/L of KXR were 0.280±0.030 and 0.430±0.040 respectively, lower significantly than that in the culture free of KXR (0.820±0.050, P < 0.01). In addition, values for MEK-1 in HSC treated with 5 mg/mL and 1.25 mg/mL of KXR were 0.143±0.013, and 0.170±0.007, respectively, being lower than that in the cells treated only with PDGF-BB (0.186±0.010, P < 0.01). The expression level of c-fos mRNA was 0.152±0.010 and 0.163±0.005, respectively, also lower than that of the PDGF group (0.183±0.014, P < 0.01).
CONCLUSION: Within the dose range used in the present study, KXR preparation shows an inhibitory effect on HSC proliferation induced by PDGF. The mechanism of this process may involve interference with Ras-MEK-MAPK singal transduction mediated by PDGF.
- Citation: Yang L, Zhu QJ, Da BH, Zhang CZ. Chinese herbs Kangxian ruangan keli inhibits expression of MEK-1 and c-fos in hepatic stellate cell indused by PDGF. Shijie Huaren Xiaohua Zazhi 2004; 12(2): 347-350
- URL: https://www.wjgnet.com/1009-3079/full/v12/i2/347.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i2.347
肝纤维化是由各种致病因子(包括慢性病毒性肝炎、酒精、药物等)导致的一种增生性疾病, 以肝星状细胞(hepatic stellate cells, HSC)的过度增生及所分泌的细胞外基质过度沉积为特征. 因此阻抑激活的肝星状细胞增生是治疗慢性肝损伤和肝纤维化的重要策略[1-6]. 血小板衍化生长因子(platelet-derived growth factor, PDGF)是HSC最强的有丝分裂原, 尤其是PDGF-BB[7-8]. Ras-MEK-MAPK信号途径是PDGF诱导肝星状细胞增生的重要胞内信号转导途径[9]. 因此阻断细胞因子的促增生作用, 则有望成为肝纤维化治疗的有效手段. 中药复方抗纤软肝颗粒具有活血化痰, 软坚散结的功效. 对于慢性肝病有良好疗效, 能防治CCl4所致的大鼠肝纤维化形成, 并能抑制HSC增生[10-15]. 我们探讨抗纤软肝颗粒对Ras-MEK-MAPK信号途径的影响如下:
肝星状细胞系(HSC-T6)由上海中医药大学徐列明教授惠赠, 其表现型为活化的HSC. 抗纤软肝颗粒由丹参、莪术、海藻、鳖甲等组成, 由湖北中医学院附属医院制备, 含生药2 g/g, 用DMEM溶解, 经0.45 µm滤器过滤除菌备用. DMEM培养基、小牛血清(fetal calf serum, FCS)美国GIBCO公司产品, PDGF-BB购自Sigma公司产品, MEK-1单抗购自Santa Cruz公司、c-fos原位杂交试剂盒购自武汉博士德生物工程有限公司, ECL试剂购自Pierce公司.
细胞培养及药物处理 HSC-T6复苏后, 接种于含100 mL/L 小牛血清, 1×10-5U/L 青霉素, 100 mg/L链霉素, 10 g/L谷氨酰胺和0.1 mmol/ L HEPES的DMEM培养基中. 在含50 mL/L CO2培养箱中于37 ℃培养. 亚单层HSC无血清培养24 h后, 加入无血清培养基稀释的抗纤软肝颗粒(设5 g/L, 1.25 g/L 2个组)作用24 h后, 加入PDGF(10 pg/L), 经24 h培养后, 再加入上述浓度的抗纤软肝颗粒, 作用3 h后再加入PDGF 10 pg/L, 5 min后将细胞消化离心. 并设空白对照组和PDGF组, 每组设4复孔, 结果取均值.
HSC-T6细胞增生的检测采用MTT比色法. 细胞按1×108 cells/. L接种于96孔培养板, 按上述药物处理后, 加入1 g/L 的MTT液20 µL, 37 ℃孵育4 h, 加入二甲基亚砜溶解结晶, 2 min后, 用全自动酶标仪测定波长570 nm处各组细胞的吸光度A值.
Western blot分析MEK-1的表达上述药物处理的细胞用冰冷的PBS洗涤2次, 加入样品裂解液[50 mmol/L Tris. CL, pH8.0, 150 mmol/L NaCl, 0.2 g/L叠氮钠, 1 g/L SDS, 100 mg/L苯甲基磺酰氟, 1 mg/L Aprotinin, 10 g/L Nonidet P-40, 5 g/L去氧胆酸钠], 置冰上孵育20 min, 4 ℃, 12 000 g, 离心2 min, 用70 g/L SDS-PAG, 每孔上样6 μg蛋白进行电泳, 电转移至硝酸纤维素膜上, MEK-1mAb 1: 400稀释, 二抗1: 2 000稀释, 应用ECL增强化学发光法显迹, X光片显影, HPIAS-1 000图像分析仪进行半定量分析.
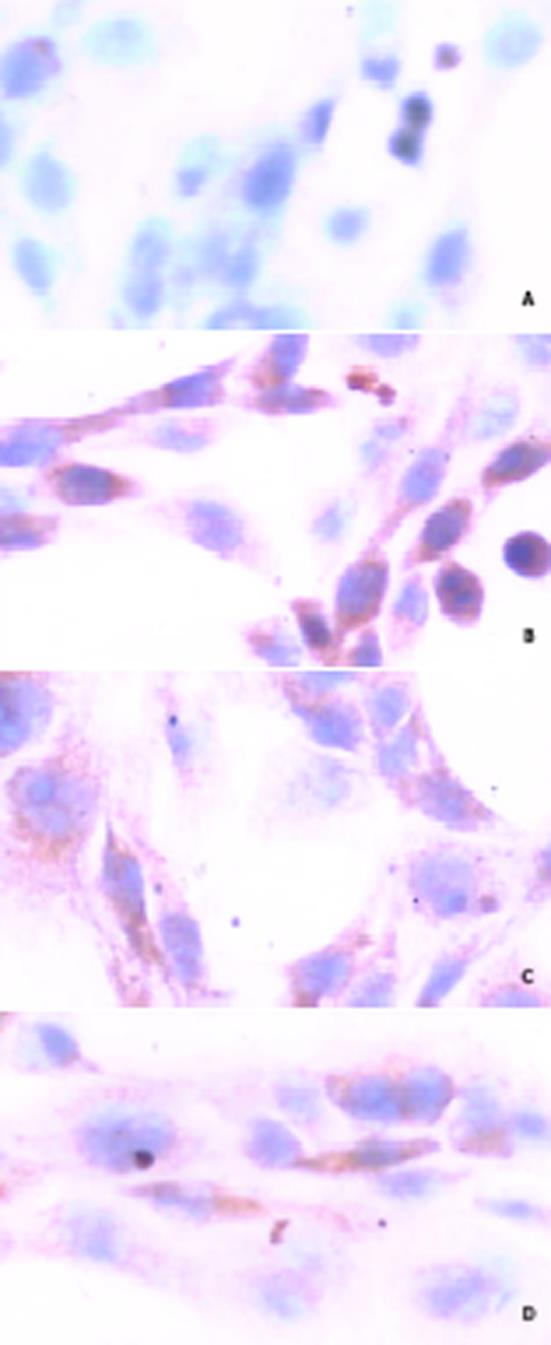
原位杂交分析c-fos mRNA的表达 以地高辛标记c-fos寡核苷酸探针进行原位杂交. 细胞爬片经40 g/L多聚甲醛固定, 5 mL/L H2O2/甲醛室温处理, 蛋白酶K消化; 每张爬片加20 µL含寡核苷酸探针的原位杂交液, 37℃, 杂交过夜; 采用过氧化物酶标记地高辛抗体-DAB显色系统显色, 阳性杂交信号呈棕黄色; 以不加含探针的原位杂交液作阴性对照, 结果为阴性; 阳性结果用HPIAS-1000高清晰度彩色病理图文分析系统进行显微图像分析 (结果以平均光密度A值表示).
统计学处理 结果用mean±SD表示, 采用t 检验, P<0.05则具有统计学差异.
抗纤软肝颗粒呈剂量依赖性抑制PDGF诱导的细胞分裂增生, 以5 g/L浓度时最为显著, 与PDGF对照组比较差异显著(P<0.01, 表1).
无血清培养的HSC经PDGF诱导后引起MEK-1表达增强, PDGF组为0.186±0.010 (图1), 而空白组为0.122±0.008, P<0.01; 经抗纤软肝颗粒作用后引起MEK-1表达呈剂量依赖性减弱, 5 g/L与1.25 g/L组MEK-1表达水平分别为0.143±0.013、0.169±0.007, 其中以抗纤软肝颗粒5 g/L组作用最显著.
为观察抗纤软肝颗粒对PDGF信号的影响, 我们进一步研究了其对MEK-1激活后的下游信号分子c-fos基因表达的影响, 结果表明与空白组(0.130±0.010, 图2A)比较, PDGF能显著增强c-fosmRNA的表达(0.183±0.014, 图2B, P<0.01), 而抗纤软肝颗粒则能显著抑制c-fos mRNA的表达, 5 g/L与1.25 g/L组c-fos mRNA的表达水平分别为0.152±0.010、图2D, 0.163±0.005, 图2C, 与PDGF组比较有显著性差异, P<0.01.
抗纤软肝颗粒中丹参具有一系列重要药理作用如抗炎、抗氧化、抑制HSC增生等[16-17], 其提取物还能诱导HSC凋亡[18-19] ; 莪术油能抑制成纤维细胞增生及肝癌增生细胞核抗原(PCNA)的表达[20-21] ; 海藻能提高机体清除具有重要致纤维化作用的活性氧的能力[22]. 该方能改善慢性肝病和肝硬化患者的肝功能, 降低血清肝纤维化指标, 并能防治CCl4诱导的肝纤维化大鼠细胞外基质的产生和沉积, 抑制体外HSC增生、分泌胶原及PDGF诱导的酪氨酸磷酸化蛋白的表达[23]. HSC被激活后获得了成纤维样细胞的表型特征: 表达α-SMA、增生活跃、细胞外基质合成增加[4,24,35]. 在这一过程中, 细胞因子及其胞内信号转导途径发挥了重要作用[9,24-29]. 近年来, 已有不少研究揭示了在肝纤维化形成过程中肝星状细胞激活的胞内信号途径, 其中PDGF诱导HSC增生的胞内机制研究得相对清楚. 因此以抑制PDGF及其信号传递为目标的治疗策略备受重视.
细胞因子与其相应受体结合后, 可启动胞质中信号转导, 通过多种途径将信号传递到胞核内, 促进或抑制特定靶基因的表达[26,28]. MEK-1是MAP Kinases的上游激酶, MAPK被激活后, 可催化c-jun、c-fos、c-myc以及核糖体S6蛋白激酶(RSK)的磷酸化, 以调节基因转录和mRNA的翻译, 使细胞由G0期进入到G1期[30-35]. 在HSC中, MAPK通路是PDGF激活c-fos表达和丝裂原作用的重要信号通路之一. PDGF受体和接头蛋白arb2的联接导致交换因子mSos的聚集, 同时激活Ras, 进一步促使Raf-1、MEK和ERK的级联激活, 而药物在MAPK活化通路中的干扰作用, 可以减低PDGF对HSC的潜在丝裂原作用[28,33].
我们采用Westernblotting方法, 分析了抗纤软肝颗粒对PDGF诱导的HSC Ras-MEK-1-MAPK信号通路的影响, 结果表明HSC中MEK-1的表达, PDGF组明显高于空白对照组及抗纤软肝颗粒各剂量组, 其中5 mg组显著低于1.25 mg组, 各组间有显著性差异. 本实验所采用的抗纤软肝颗粒的浓度是经预实验获得的, 并在安全范围内, 细胞成活率在95%以上. 因此所观察到的以上结果具有一定的特异性, 而与药物的细胞毒作用无关. 进一步研究了抗纤软肝颗粒对MEK-1激活后的下游信号分子c-fos基因表达的影响, 表明PDGF能显著增强c-fos mRNA的表达, 而抗纤软肝颗粒则能显著抑制c-fos基因的表达. c-fos基因是PDGF丝裂原信号经胞质传递至核内引起细胞增生的转录因子. 结合前期研究表明抗纤软肝颗粒可能通过抑制酪氨酸磷酸化蛋白的表达, 进而使HSC内酪氨酸激酶活性水平降低, 导致下游的蛋白激酶水平(如MEK-1)下调, 核内增生信号减弱, 从而使细胞的生长状态受到抑制, 这可能是该方抑制肝星状细胞增生的作用机制之一.
编辑: N/A
| 1. | Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129-140. [PubMed] [DOI] |
| 3. | Wang JY, Zhang QS, Guo JS, Hu MY. Effects of glycyrrhetinic acid on collagen metabolism of hepatic stellate cells at different stages of liver fibrosis in rats. World J Gastroenterol. 2001;7:115-119. [PubMed] [DOI] |
| 4. | Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808-d826. [PubMed] [DOI] |
| 5. | Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2-13. [PubMed] [DOI] |
| 6. | Kinnman N, Goria O, Wendum D, Gendron MC, Rey C, Poupon R, Housset C. Hepatic stellate cell proliferation is an early platelet-derived growth factor-mediated cellular event in rat cholestatic liver injury. Lab Invest. 2001;81:1709-1716. [PubMed] [DOI] |
| 7. | Iwamoto H, Nakamuta M, Tada S, Sugimoto R, Enjoji M, Nawata H. Platelet-derived growth factor receptor tyrosine kinase inhibitor AG1295 attenuates rat hepatic stellate cell growth. J Lab Clin Med. 2000;135:406-412. [PubMed] [DOI] |
| 8. | Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437-451. [PubMed] [DOI] |
| 9. | Marra F, Arrighi MC, Fazi M, Caligiuri A, Pinzani M, Romanelli RG, Efsen E, Laffi G, Gentilini P. Extracellular signal-regulated kinase activation differentially regulates platelet-derived growth factor's actions in hepatic stellate cells, and is induced by in vivo liver injury in the rat. Hepatology. 1999;30:951-958. [PubMed] [DOI] |
| 11. | 杨 玲, 朱 清静, 张 赤志. 抗纤软肝冲剂药物血清对激活肝星状细胞表达Ⅰ型前胶原及TGF-β1mRNA的影响. 中国中医基础医学杂志. 2001;7:38-40. |
| 15. | 杨 玲, 张 赤志, 朱 清静, 杨 胜兰. 抗纤软肝颗粒对肝星状细胞增生的影响. 中国中西医结合消化杂志. 2002;10:323-324. |
| 16. | Liu CH, Liu P, Hu YY, Xu LM, Tan YZ, Wang ZN, Liu C. Effects of salvianolic acid-A on rat hepatic stellate cell proliferation and collagen production in culture. Acta Pharmacol Sin. 2000;21:721-726. [PubMed] |
| 17. | Liu P, Liu CH, Wang HN, Hu YY, Liu C. Effect of salvianolic acid B on collagen production and mitogen-activated protein kinase activity in rat hepatic stellate cells. Acta Pharmacol Sin. 2002;23:733-738. [PubMed] |
| 18. | Zhang XL, Liu L, Jiang HQ. Salvia miltiorrhiza monomer IH764-3 induces hepatic stellate cell apoptosis via caspase-3 activation. World J Gastroenterol. 2002;8:515-519. [PubMed] [DOI] |
| 19. | Yao XX, Tang YW, Yao DM, Xiu HM. Effects of Yigan Decoction on proliferation and apoptosis of hepatic stellate cells. World J Gastroenterol. 2002;8:511-514. [PubMed] [DOI] |
| 23. | Yang L, Zhang CZ, Zhu QJ. Kangxian ruangan keli inhibits hepatic stellate cell proliferation mediated by PDGF. World J Gastroenterol. 2003;9:2050-2053. [PubMed] [DOI] |
| 24. | Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397-416. [PubMed] [DOI] |
| 25. | Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427-436. [PubMed] [DOI] |
| 26. | Britton RS, Bacon BR. Intracellular signaling pathways in stellate cell activation. Alcohol Clin Exp Res. 1999;23:922-925. [PubMed] [DOI] |
| 27. | Maeda N, Kawada N, Seki S, Arakawa T, Ikeda K, Iwao H, Okuyama H, Hirabayashi J, Kasai K, Yoshizato K. Stimulation of proliferation of rat hepatic stellate cells by galectin-1 and galectin-3 through different intracellular signaling pathways. J Biol Chem. 2003;278:18938-18944. [PubMed] [DOI] |
| 28. | Pinzani M. PDGF and signal transduction in hepatic stellate cells. Front Biosci. 2002;7:d1720-d1726. [PubMed] [DOI] |
| 29. | Liu XJ, Yang L, Mao YQ, Wang Q, Huang MH, Wang YP, Wu HB. Effects of the tyrosine protein kinase inhibitor genistein on the proliferation, activation of cultured rat hepatic stellate cells. World J Gastroenterol. 2002;8:739-745. [PubMed] [DOI] |
| 30. | Carloni V, Defranco RM, Caligiuri A, Gentilini A, Sciammetta SC, Baldi E, Lottini B, Gentilini P, Pinzani M. Cell adhesion regulates platelet-derived growth factor-induced MAP kinase and PI-3 kinase activation in stellate cells. Hepatology. 2002;36:582-591. [PubMed] [DOI] |