修回日期: 2004-10-20
接受日期: 2004-10-27
在线出版日期: 2004-12-15
目的: 探讨血液滤过减轻急性重症胰腺炎(SAP)全身炎症反应的机制.
方法: 逆行性胰管注射50 g/L牛黄胆酸钠/自身胆汁制备犬SAP动物模型, 2 h后实施血液滤过2 h, 并设立对照组(制模后仅给予深静脉穿刺插管而不血液滤过). 记录不同时间段心率, 12 h处死动物后通过病理学评分评价肺、肝脏和胰腺组织损伤情况, Westen blotting法检测肺和肝脏组织核转录因子NF-κB的核移位及RT-PCR检测肿瘤坏死因子TNF-αmRNA表达, 通过比较血滤组和对照组脏器组织炎性激活和损伤的情况, 分析血液滤过的作用机制.
结果: 血滤前心率两组之间无显著差异, 血滤后各时间点均为对照组显著高于血滤组. 病理学评分显示血滤减轻肺脏的损伤, 而对肝脏和胰腺损伤作用不明显. NF-κB核移位和TNF-αmRNA表达均为血滤组低于对照组.
结论: 血液滤过减轻肺、肝脏等远离病灶器官的炎症激活和损伤, 这一作用可能同血液中致炎因子的滤过清除有关.
引文著录: 李磊, 汤耀卿, 毛恩强, 秦帅, 陈胜, 张明钧. 急性重症胰腺炎血液滤过治疗的机制. 世界华人消化杂志 2004; 12(12): 2822-2825
Revised: October 20, 2004
Accepted: October 27, 2004
Published online: December 15, 2004
AIM: To study the mechanism of hemofiltration in the reduction of systemic inflammatory response in severe acute pancreatitis (SAP).
METHODS: A mixture composed of sodium taurocholate (50 g/L) and bile was antidromicly injected into pancreatic duct of dogs to establish SAP model. Two hours later, hemofiltration was performed (last 2 h). Heart rate, as well as the acute injury scores of lung, liver and pancreas were compared between model and test groups. Nuclear translocation of nuclear factor-κB (NF-κB) was detected by Western blotting, and TNF-α mRNA expression was determined by reverse transcription-polymerase chain reaction. Then the therapeutic mechanism of hemofiltration in SAP was analyzed.
RESULTS: Significant decrease of heart rates was observed 8 and 12 h after the hemofiltration (P = 0.0 181 < 0.05, P = 0.0 141 < 0.05 respectively). Hemofiltration resulted in reduction of pulmonary pathological score (1 ± 0.63 vs 2.83 ± 0.75, P = 0.001 < 0.01), but did not affect hepatic and pancreatic ones. NF-κB nuclear translocation and TNF-α expression were inhibited by hemofiltration both in lung and in liver.
CONCLUSION: Hemofiltration ameliorates pulmonary and hepatic inflammatory response induced by SAP. This may relate to removal of the over-produced pro-inflammatory cytokines from circulation.
- Citation: Li L, Tang YQ, Mao EQ, Qin S, Chen S, Zhang MJ. Mechanism of hemofiltration in treatment of severe acute pancreatitis. Shijie Huaren Xiaohua Zazhi 2004; 12(12): 2822-2825
- URL: https://www.wjgnet.com/1009-3079/full/v12/i12/2822.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i12.2822
血液滤过(hemofiltration, HF)可以缓解急性全身炎症反应综合征(SIRS)病情, 在急性重症胰腺炎(SAP)等危重疾病治疗中的应用价值已经得到广泛的认可[1-4]. 一般认为这种作用同血液滤过清除血液循环中的炎症递质有关[5]. 但目前尚没有研究进一步探讨血液滤过对远离原发病灶器官炎症激活和损伤的影响. 我们观察血液滤过对犬SAP时肺脏和肝脏的保护作用, 就血液滤过的作用机制进行进一步探索.
杂种犬12只, 质量体重20-30 kg, 雌雄兼有, 无基础病变. 急性重症胰腺炎制模前12 h禁食, 自由饮水; im盐酸氯安酮0.2 g, 阿托品0.5 mg诱导麻醉, iv 25 g/L异戊巴比妥维持麻醉. 气管插管, 连接心电监护仪, 开放静脉通路, 必要时用呼吸机;取上腹部正中切口进入腹腔, 找到胆囊后抽取动物自身胆汁5 mL; 纵行切开十二指肠降部对系膜面, 寻找主乳头开口, 将一硬膜外麻醉管插入主胰管内约5 cm, 然后加压注入50 g/L牛黄胆酸钠0.7 mL/kg和自身胆汁2-3 mL混合液, 注射时间为20 s, 停留5 min后拔出麻醉管, 缝合十二指肠壁; 行膀胱造漏置管; 关闭腹部切口. 动物共分两组, 每组6只: 对照组制模后给予常规补液、呼吸机等处理, 维持各项生命体征稳定; 血滤组除给予补液、呼吸机等处理外, 制模2 h后开始血液滤过.
采用宁波亚泰医疗有限公司出品的YT-120聚砜血滤器, 普通肝素抗凝, 前置换方式补充置换液. 置换液流速: 2 000 mL/h(1 700-2 400 mL/h); 血液流速: 180 mL/h(160-200 mL/h); 超滤速度: 50 mL/h; 治疗时间: 2 h. 记录心电监护设备显示各时间点数值; 12 h处死动物后收集腹水并计算腹水量(mL/kg). 处死动物后取出胰腺、肝脏、肺脏以及肾脏组织, 40 g/L甲醛保存; 病理组织石蜡包埋和HE染色由瑞金医院病理科完成; 病理学评分由本院病理科医生盲态下完成. 评分标准参照文献[6-8]. 待测脏器组织取出后立刻置于冻存管并在液氮中保存; 检测时取组织约150 mg, 加入匀浆缓冲液A0.5 mL, 冰中匀浆10 min, 取上清液; 10 000 g离心10 min, 去除上清液后加入匀浆缓冲液B混匀, 10 000 g离心2 min, 取上清液为核内容物, -70 ℃保存. 配制分离胶和积层胶, 样品变性, 加样, 每个加样孔加样15 uL(总蛋白量100 ug), 80V SDS-PAGE电泳; 电泳后将条带转移到硝酸纤维膜上, 去离子水洗膜, 20 g/L白蛋白常温封闭1 h, 分别加入1:500的一抗和二抗(美国Santa cruz公司), NBT/BCIP显色. 免疫印迹法(Western blotting)检测组织细胞核内NF-κB蛋白总量. RT-PCR比较IL-10和TNF-α组织中表达IL-10上游引物和下游引物分别为5'-GAC AAG CTG GAC AAC ATA CTG CTG ACC-3'和5'-TCC TAG AGT CGA GAA GAG TTG CCA TCC-3'; TNF-α的上游引物和下游引物分别为5'-ACT CTT CTG CCT GCT GCA CTT TGG-3'和5'-GTT GAC CTT TGT CTG GTA GGA GAC GG-3'内参照G3PDH的上游引物和下游引物分别为5'-GAA CGG GAA GCT CAC TGG CAT GGC-3'和5'-TGA GGT CCA CCA CCC TGT TGC TG-3'. 引物由上海生工生物工程研究所合成. TRIzol(美国GIBCO公司)法抽提总RNA, 步骤参照试剂盒提供方法进行. 抽提的RNA经紫外分光光度仪检测A260/A280>1.8. 一步法RT-PCR按照试剂盒(美国Promega公司)说明进行扩增. 产物于20 g/L琼脂糖凝胶中电泳30 min, 计算机扫描.
统计学处理 计量资料以mean±SD表示; 数据比较采用团体t检验; P<0.05有统计学意义.
血滤前(制模后2 h)两组心率无显著差异(P>0.05), 制模后4 h HF组较NHF组低但无显著差异(P = 0.0515), 8 h和12 h均是HF组显著低于NHF组(P = 0.0 181和0.0 141). HF组和NHF组动物腹水量分别为31.1±5.6和12.7±2.9, 两组间有显著性差异(P = 0.001).
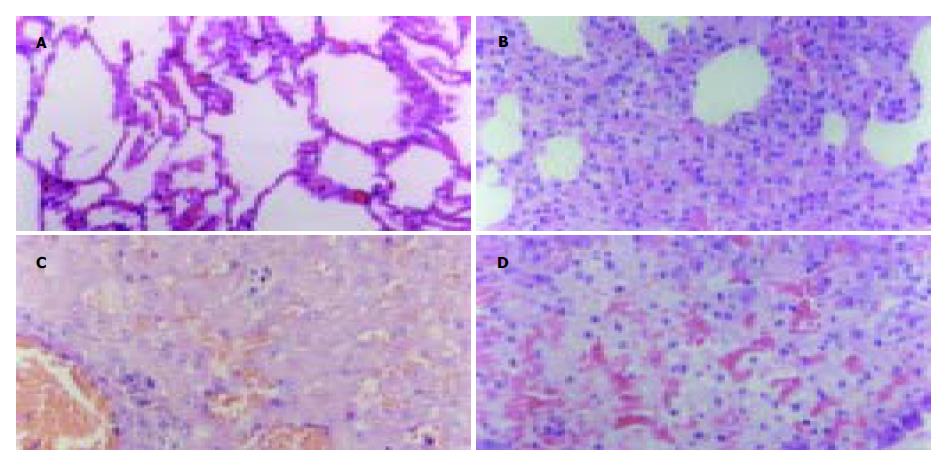
HF组肺脏急性损伤评分显著低于NHF组(1±0.63 vs 2.83±0.75, P = 0.001, 图1A, B); 肝(2.17±0.75 vs 2.83±0.75, 图1C)和胰(10±1.30 vs 10.83±1.78, 图1D)急性损伤评分两组无显著差异(P = 0.1 561和0.3 766).
我们曾发现短时血液滤过显著降低SAP患者血液TNF-α、IL-1β、IL-6、8等促炎细胞因子水平, 而以IL-10为主的抗炎细胞因子却呈上升趋势[9]. 全身炎症反应过度激活造成肺、肝脏及肾脏等远离胰腺病灶器官的炎性损伤是多脏器功能障碍综合征(MODS)发生的直接原因. 在本研究中我们观察到: 在血液滤过过程中, SAP犬的心率呈下降的趋势, 在血滤停止后轻度上升, 而对照组在制作模型后全程呈现上升的趋势, 且在4, 8和12 h心率对照组均较血滤组高, 其中8和12 h两组之间差异显著. 腹水的量是判断腹膜炎严重程度的一项重要指标. 血滤组的腹水/kg比值显著低于对照组, 说明血液滤过能减轻腹膜炎程度. 我们还通过病理学评分比较了制模12 h后两组动物肺、肝和胰腺的急性损伤情况, 结果显示血液滤过减轻远隔脏器, 特别是肺脏的损伤. 以上结果表明, 血液滤过达到减轻全身炎症反应和远离胰腺病灶器官炎症反应的目的. 血液滤过减轻急性胰腺炎初期全身炎症反应程度可能同清除了血液中的促炎递质有关[5,10-11]. TNF-α, IL-1, 6等促炎细胞因子的分子量集中在Mr 10 000-30 000, 而半透膜滤过孔径的上限是Mr 30 000[12], 这样通过过度释放入血的细胞因子可能通过滤过膜的微孔而被清除出体外. 为增加炎性物质的清除效率, 近年来的多项研究采用多粘菌素B纤维血液灌流和活性炭血液灌流等方法, 取得了更加显著的效果[13-14].
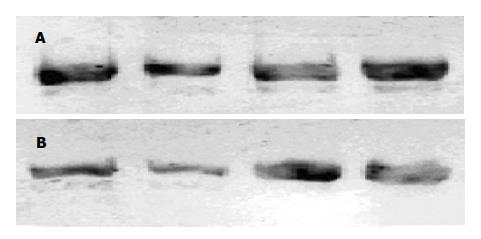
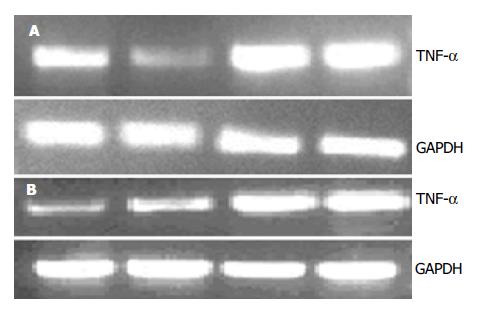
在本研究我们观察血液滤过是否能影响到远离病灶器官的炎症反应激活程度. Westen blot法检测肺脏和肝脏组织细胞核提取物核转录因子NF-κB含量显示血滤组低于对照组; 半定量RT-PCR检测肝脏和肺脏组织TNF-α表达同样显示血滤组低于对照组. NF-κB是促炎细胞因子的总开关, 参与调节TNF-α, IL-1, 6, 8以及NOS, 黏附因子等大多数重要炎症递质mRNA转录[15-16]. 其水平下降说明血液滤过能减轻远隔脏器的炎症反应程度. 由此, 我们进一步提出SAP全身炎症反应激活和血液滤过的作用机制是: SAP早期胰腺坏死局部产生和释放过多的促炎物质入血是引起全身炎症反应的根源, 可能包括细胞因子和其他中、小分子物质, 通过血液滤过清除这些物质能减轻远隔脏器的炎症反应激活. 在SAP起病早期最先出现损害的脏器常是肺[17]. 在本研究中我们观察到血液滤过对肺的保护作用最为明显. 肝脏是受累最早的器官之一, 但表现出受损的时间要迟于肺. 发病12 h后肝脏损害可能还不能充分表现出来, 因此虽然处理组和对照组之间病理学评分差别不大, 但NF-κB和TNF-α水平已经有显著差异.
胰腺作为原发病灶, 血液滤过对其影响不大, 血滤组和对照组均观察到明显坏死. 原因可能是血液滤过对胰腺原发病灶局部促炎物质清除作用有限. 但血滤组腹水显著少于对照组, 证实胰性腹水不仅仅是局部胰腺坏死渗出刺激的结果, 还是全身炎症反应的一部分, 血液中炎症递质的清除有助于减轻腹膜炎程度.
编辑: N/A
| 1. | Yekebas EF, Treede H, Knoefel WT, Bloechle C, Fink E, Izbicki JR. Influence of zero-balanced hemofiltration on the course of severe experimental pancreatitis in pigs. Ann Surg. 1999;229:514-522. [PubMed] [DOI] |
| 2. | Wang H, Li WQ, Zhou W, Li N, Li JS. Clinical effects of continuous high volume hemofiltration on severe acute pancreatitis complicated with multiple organ dysfunction syndrome. World J Gastroenterol. 2003;9:2096-2099. [PubMed] [DOI] |
| 3. | Kellum JA, Song M, Venkataraman R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit Care Med. 2004;32:801-805. [PubMed] [DOI] |
| 4. | Oliver WC Jr, Nuttall GA, Orszulak TA, Bamlet WR, Abel MD, Ereth MH, Schaff HV. Hemofiltration but not steroids results in earlier tracheal extubation following cardiopulmonary bypass: a prospective, randomized double-blind trial. Anesthesiology. 2004;101:327-339. [PubMed] [DOI] |
| 5. | Teraoka S, Mineshima M, Hoshino T, Ishimori I, Kaneko I, Sato Y, Haruguchi H, Agishi T. Can cytokines be removed by hemofiltration or hemoadsorption? ASAIO J. 2000;46:448-451. [PubMed] [DOI] |
| 6. | Camargo CA Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513-1520. [PubMed] [DOI] |
| 7. | Kiyonari Y, Nishina K, Mikawa K, Maekawa N, Obara H. Lidocaine attenuates acute lung injury induced by a combination of phospholipase A2 and trypsin. Crit Care Med. 2000;28:484-489. [PubMed] [DOI] |
| 8. | Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56. [PubMed] [DOI] |
| 9. | Mao EQ, Tang YQ, Zhang SD. Formalized therapeutic guideline for hyperlipidemic severe acute pancreatitis. World J Gastroenterol. 2003;9:2622-2626. [PubMed] |
| 10. | Su X, Bai C, Hong Q, Zhu D, He L, Wu J, Ding F, Fang X, Matthay MA. Effect of continuous hemofiltration on hemodynamics, lung inflammation and pulmonary edema in a canine model of acute lung injury. Intensive Care Med. 2003;29:2034-2042. [PubMed] [DOI] |
| 11. | De Vriese AS, Colardyn FA, Philippé JJ, Vanholder RC, De Sutter JH, Lameire NH. Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol. 1999;10:846-853. [PubMed] |
| 12. | Schetz M, Ferdinande P, Van den Berghe G, Verwaest C, Lauwers P. Removal of pro-inflammatory cytokines with renal replacement therapy: sense or nonsense? Intensive Care Med. 1995;21:169-176. [PubMed] [DOI] |
| 13. | Kodama M, Hanasawa K, Tani T. Blood purification for critical care medicine: endotoxin adsorption. Ther Apher. 1997;1:224-227. [DOI] |
| 14. | Winchester JF, Salsberg JA. Sorbents in the treatment of renal failure. Minerva Urol Nefrol. 2004;56:215-221. [PubMed] |
| 15. | Paterson RL, Galley HF, Dhillon JK, Webster NR. Increased nuclear factor kappa B activation in critically ill patients who die. Crit Care Med. 2000;28:1047-1051. [PubMed] [DOI] |
| 16. | Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31:S105-S111. [PubMed] [DOI] |