修回日期: 2004-09-25
接受日期: 2004-09-30
在线出版日期: 2004-12-15
目的: 了解STAT3和TEC在肝再生以及HGF刺激的肝干细胞中激活情况, 探讨在肝细胞早期增生中STAT3和TEC的活化的相关性.
方法: 建立小鼠肝大部分切除和HGF刺激肝干细胞(WB F-344)的体内、体外两个试验模型, 采用免疫沉淀(immun-oprecipitation, IP)、免疫印迹(immunoblotting, IB)的方法检测TEC和STAT3酪氨酸磷酸化激活水平与时间, 使用凝胶阻滞实验(electrophoretic mobility shift assay, EMSA)分析核蛋白与STAT3 DNA特异序列的结合能力.
结果: 肝大部分切除和HGF刺激下STAT3和TEC的磷酸化水平均快速明显升高, 肝大部分切除后10-20 min时二者激活水平均达到最高, HGF刺激后10 min TEC激活水平最高, 30 min STAT3活化水平最高. 肝大部分切除或HGF刺激下10 min左右, 核蛋白与STAT3 DNA特异序列的结合能力明显增强.
结论: 肝大部分切除和HGF刺激下STAT3和TEC均被快速激活, 他们之间可能存在相互作用, 共同影响肝细胞早期增生反应.
引文著录: 李菲菲, 郑红, 许望翔, 杨晓明, 汪思应. 肝大部分切除或HGF刺激可以引起STAT3和TEC的同时激活. 世界华人消化杂志 2004; 12(12): 2809-2812
Revised: September 25, 2004
Accepted: September 30, 2004
Published online: December 15, 2004
AIM: To study the activation of TEC and STAT3 in the hepatocyte after partial hepatectomy (PH) or hepatocytic growth factor (HGF) stimulation in the mice.
METHODS: Mice of SPF degree and WB F-344 cell (liver stem cell line) were used in this study. In vivo and in vitro experimental models of PH and HGF stimulation were established respectively. Immunoprecipitation (IP) and immunoblotting (IB) were used to observe the phosphorylation level and time of TEC and STAT3. On the other hand, electrophoretic mobility shift assay (EMSA) was used to detect the binding ability of STAT3 DNA.
RESULTS: TEC and STAT3 were both inducibly phosphorylated in one hour after PH or HGF stimulation. Ten to twenty minutes after PH, levels of TEC and STAT3 reached the peak. About 10 min after HGF stimulation, TEC phosphorylation level reached maximum value and about 30 min STAT3 phosphorylation level reached peak value. Meanwhlie, STAT3 DNA binding activity was enhanced both In vivo and in vitro experiments.
CONCLUSION: After PH or HGF-stimulation, both TEC and STAT3 are quickly phosphorylated in one hour, and they synergically affect the early proliferation of hepatocytes.
- Citation: Li FF, Zheng H, Xu WX, Yang XM, Wang SY. Activation of TEC and STAT3 after partial hepatectomy or hepatocytic growth factor stimulation. Shijie Huaren Xiaohua Zazhi 2004; 12(12): 2809-2812
- URL: https://www.wjgnet.com/1009-3079/full/v12/i12/2809.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i12.2809
STAT3(singal transducer and activator transcription factor 3, STAT3)对不同组织的发育和细胞的增生有着至关重要的作用[1-3]. 他可以与急性期基因启动子上的增强元件(即急性期反应元件)相互作用, 对HGF, EGF等生长因子刺激诱导的肝细胞早期增生有重要作用[4-6]. TEC(tyrosine kinase expressed in hepacelluar carcinoma, TEC)是一种重要的非受体型酪氨酸激酶[7], 参与造血细胞生长、分化的调控[8], 被认为具有造血组织及肝组织分布相对特异性[9]. 我们发现大鼠TEC的mRNA在2/3肝切除的大鼠再生肝中表达迅速升高, 并发现TEC是一种与肝再生调控密切相关的早期反应基因, 他可能参与肝细胞早期生长[10-11]. 肝细胞生长因子(hepatocyte growth factor, HGF)是一种多功能的细胞活性因子, 是目前公认的最重要的肝再生启动因子之一[12]. 无论是肝再生还是HGF刺激下引起的肝细胞的增生的机制目前还不十分明了. 由于TEC与STAT3都与肝大部分切除后细胞早期增生有关, 提示我们他们二者可能共同作用于肝干细胞或是存在cross-talk. 因此我们研究STAT3和TEC在肝再生以及HGF刺激的肝干细胞中激活的情况, 探讨在肝细胞早期增生中STAT3和TEC的相关性.
鼠重组肝细胞生长因子(mHGF)为Sigma公司产品. TEC抗体、抗磷酸化PY99抗体、STAT3抗体、磷酸化STAT3抗体购自Santa Cruz. Protein A-Sepharose 4B Beads, 辣根过氧化物酶标记的IgG抗体, ECL(enhanced chemiluminescence, ECL)试剂盒购于北京中山生物公司. PVDF(polyvinylidene difluoride, PVDF)膜为Amersham公司产品. DMEM胰酶为Gibco公司产品, 胎牛血清为Life Technologies公司产品. 昆明鼠购于军事医学科学院动物实验中心. 肝干细胞[13]WB F-344为中国医学科学院药理所韩锐教授赠送. 培养条件: 高糖DMEM, 含100 g/L胎牛血清(fetal bovine serum, FBS), 37 ℃, 50 mL/L CO2孵箱培养. 5×108 /L细胞密度下使用10 g/L胎牛血清的DMEM饥饿培养24-48 h, 再用HGF刺激(20-50 μg/L). 25-30 g SPF级(♀)昆明鼠12只, 随机分为实验组与对照组, 10 g/L戊巴比妥ip麻醉后, 实验组依次进行2/3肝大部切除, 分别在切除后10, 20, 30, 60 min取再生肝组织, 以小号研磨器研磨后过滤, 加入RIPA(975 g/L PBS, 10 g/L Nonidet P-40, 5 g/L脱氧胆酸钠, 1 g/L SDS)冰上裂解30 min, 4 ℃离心取上清, 即为再生肝总蛋白. 对照组开腹牵拉肝组织后10, 20, 30, 60 min取肝组织提总蛋白.
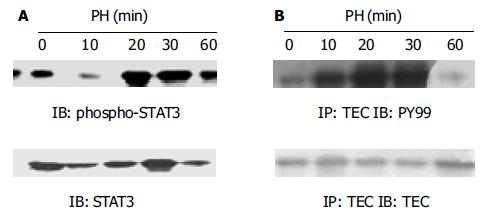
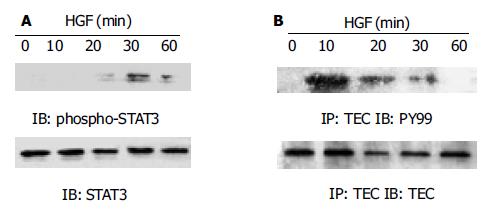
1.2.1 TEC与STAT3活化的检测: TEC酪氨酸磷酸化水平检测: 再生肝组织或HGF刺激后的细胞用冰PBS洗3遍, RIPA裂解后提取蛋白. 取细胞蛋白或组织蛋白2 mg/group, 加入抗TEC抗体(1:100稀释)在旋转器上4 ℃混匀1 h, 再加入Protein A-Sepharose 4B Beads于4 ℃混匀过夜, 将免疫沉淀复合物用冰RIPA洗4遍, 重悬于2×SDS上样缓冲液中, 100 ℃变性3 min后上样进行100 g/L SDS-PAGE电泳, 再电转(100 mA、120 min)至PVDF膜上, 转好的膜放在TBS-T(20 mmoL/L Tris-HCl PH7.4, 150 mmoL/L NaCl, 0.5 g/L Tween20)中封闭3 h, 然后再将膜置于加入抗PY99抗体(1:1 000)的TBST中室温孵育1 h, 再加入相应结合的二抗后与ECL发光检测剂结合显示结果. STAT3酪氨酸磷酸化水平检测: 再生肝组织蛋白或HGF刺激后的细胞裂解物(10 μg/group)经100 g/L SDS-PAGE胶分离后电转(100 mA, 120 min)至PVDF膜上, 转好的膜放在TBST中封闭3 h. 然后再将膜置于加入抗STAT3抗体(1:2 000)或phospho-STAT3(1:2 000)的TBST中室温孵育1 h, 再加入相应结合的二抗后与ECL发光检测剂结合显示结果.
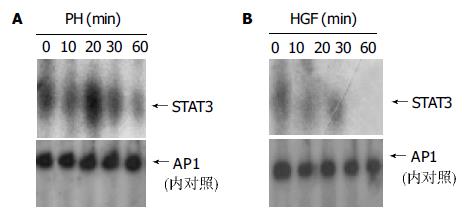
1.2.2 EMSA法检测STAT3结合活性: 核蛋白的提取: 组织提取物或细胞用预冷的PBS 10 mL洗2次, 重悬于400 μL buffer A(10 mmol/L Hepes pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.5 mmol/L DTT, 0.5 mmol/L PMSF, 1 mg/L leupeptin, 1 mg/L aprotinin, 1 mg/L pepstatin A). 冰上放置15 min, 加入12.5 μL 100 g/L Nonidet P-40, 轻轻混匀后于4 ℃ 2 000 g离心10 min, 沉淀重悬于40 μL buffer C(20 mmol/L Hepes pH 7.9, 1.5 mmol/L MgCl2, 450 mmol/L NaCl, 250 g/L glycerol, 0.2 mmol/L EDTA, 0.5 mmol/L DTT, 0.5 mmol/L PMSF, 1 mg/L leupeptin, 1 mg/L aprotinin, 1 mg/L pepstatin A), 冰上静置30 min, 20 000 g离心15 min, 上清即为核提取物. STAT3同源寡核苷酸片段为Promega公司标记和纯化产品. 探针的制备: 32P标记STAT3寡核苷酸(1.75p mol/L)2 μL, 10×T4缓冲液1 μL, 32P-ATP(111PBq/mmol)1 μL, 无菌水5 μL, T4核酸酶(5-10 MU/L)1 μL, 37 ℃反应10 min, 加入1 μL 0.5 mol/L EDTA终止反应. EMSA实验: 98 μg核蛋白在结合液(20mmol/L Hepes pH 7.9, 100 mmol/LKCl, 200 g/L glycerol, 0.2 mmol/L EDTA, 0.5 mmol/L DTT, 0.5 mmol/L PMSF)中与1 μL 32P标记STAT3寡核苷酸中4 ℃反应15 min. 加上样缓冲液1 μL, 上样, DNA-protein复合物在400 g/L聚丙酰胺凝胶中电泳(300V, 30-40 min), 取出凝胶压片. 在-20 ℃放射性自显影8-24 h.
肝大部分切除后STAT3与TEC磷酸化水平均在1 h内迅速升高, 其中STAT3激活水平在10-20 min达最高(图1A), TEC激活水平在20-30 min达最高(图1B).
在HGF刺激下, 肝干细胞WB F-344中STAT3和TEC快速激活. 其中在TEC在HGF刺激10 min时磷酸化水平达最高(图2A), STAT3则在30 min时磷酸化水平达最高(图2B).
肝大部分切除和HGF刺激1 h内, 核蛋白与STAT3的DNA结合能力呈瞬时性增强, 在肝大部分切除20 min左右, HGF刺激10 min左右结合力达最高(图3).
本结果表明, 在肝切除或生长因子等多种因素刺激下, TEC和STAT3被迅速激活, 都共同参与了肝细胞的增生反应, 可能对肝细胞的早期分裂增生发挥重要作用. STAT3参与了多种病理生理过程中的应答, 是gp-130受体(IL-6受体)下游最重要的介导信号转导的分子[14-15]. STAT3和其他STAT蛋白一样, 在氨基末端有1个4聚体化区, 参与受体的募集与STAT自身二聚体化. STAT3的酪氨酸磷酸化是在细胞因子刺激下, 由JAK激酶介导下而发生的[16-17], 这种酪氨酸磷酸化对STAT3的二聚体化激活、核定位、与DNA结合是必须的[18]. TEC基因编码框由PH domain、TH domain、SH3 domain、SH2 domain和kinase domain组成[19]. SH2 domain与kinase domain可以与下游一些蛋白DokI, BROG1, PKC-等结合, 从而使后者磷酸化而激活[20-22].
肝再生是一个复杂的病理生理过程, 有很多基因参与其中[23-24]. 以往的研究表明STAT3与TEC是肝再生早期反应基因[25-27]. HGF是肝再生最强的启动因子之一[28-29]. HGF与其细胞表面的受体c-met结合后, 引起细胞内一系列蛋白酪氨酸磷酸化[30]. 我们有理由设想STAT3与TEC间可能也存在交叉对话, 共同影响肝细胞早期增生反应. 根据我们的实验结果, 我们提出TEC在活化后可以使STAT3活化的假设, 有两种可能, 一种简单的假设是TEC磷酸化激活后发挥其激酶活性, 其kinase domain使STAT3的碳末端活化区的酪氨酸位点磷酸化, 直接激活STAT3; 另一种可能是间接激活, TEC活化后, 通过激活其他激酶使STAT3发生磷酸化活化, 这种激酶可能是JAK2或者是TEC下游其他靶基因. TEC与STAT3激活后将调节一些对细胞增生起重要作用的基因的活性, 如c-fos, 从而调节细胞增生信号转导途径, 最终对急性肝损伤或细胞因子诱导下的肝细胞有丝分裂起正相调控作用. 本研究为进一步探讨STAT3与TEC之间信号转导关系奠定了基础, 为进一步深入了解肝细胞再生机制提供了新思路.
编辑: N/A
| 2. | Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143-1148. [PubMed] [DOI] |
| 4. | Leu JI, Crissey MA, Leu JP, Ciliberto G, Taub R. Interleukin-6-induced STAT3 and AP-1 amplify hepatocyte nuclear factor 1-mediated transactivation of hepatic genes, an adaptive response to liver injury. Mol Cell Biol. 2001;21:414-424. [PubMed] [DOI] |
| 5. | Zhong Z, Wen Z, Darnell JE Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95-98. [PubMed] [DOI] |
| 6. | Lütticken C, Wegenka UM, Yuan J, Buschmann J, Schindler C, Ziemiecki A, Harpur AG, Wilks AF, Yasukawa K, Taga T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994;263:89-92. [PubMed] [DOI] |
| 7. | Tsygankov AY. Non-receptor protein tyrosine kinases. Front Biosci. 2003;8:s595-s635. [PubMed] [DOI] |
| 8. | Lucas JA, Miller AT, Atherly LO, Berg LJ. The role of Tec family kinases in T cell development and function. Immunol Rev. 2003;191:119-138. [DOI] |
| 9. | Mano H, Ishikawa F, Nishida J, Hirai H, Takaku F. A novel protein-tyrosine kinase, tec, is preferentially expressed in liver. Oncogene. 1990;5:1781-1786. [PubMed] |
| 10. | Xu W, Wang S, Wang G, Wei H, He F, Yang X. Identification and characterization of differentially expressed genes in the early response phase during liver regeneration. Biochem Biophys Res Commun. 2000;278:318-325. [PubMed] [DOI] |
| 11. | 汪 思应, 王 阁, 许 望翔, 魏 汉东, 杨 晓明. Tec酪氨酸蛋白激酶基因是一种与肝再生调控相关的早期反应基因. 中国生物化学与分子生物学报. 2001;17:325-328. |
| 12. | Gohda E. Function and regulation of production of hepatocyte growth factor (HGF). Nippon Yakurigaku Zasshi. 2002;119:287-294. [DOI] |
| 14. | Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548-2556. [PubMed] [DOI] |
| 15. | Schuringa JJ, Jonk LJ, Dokter WH, Vellenga E, Kruijer W. Interleukin-6-induced STAT3 transactivation and Ser727 phosphorylation involves Vav, Rac-1 and the kinase SEK-1/MKK-4 as signal transduction components. Biochem J. 2000;347 Pt 1:89-96. [PubMed] [DOI] |
| 16. | Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274:16513-16521. [PubMed] [DOI] |
| 17. | Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F, Pellegrini S, Yasukawa K, Heinrich P, Stark GR. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 1995;14:1421-1429. [PubMed] |
| 18. | Takeda K, Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev. 2000;11:199-207. [DOI] |
| 19. | Mano H. Tec family of protein-tyrosine kinases: an overview of their structure and function. Cytokine Growth Factor Rev. 1999;10:267-280. [DOI] |
| 20. | Mayer BJ, Hirai H, Sakai R. Evidence that SH2 domain promote processive phosphorylation by protein-tyrosine kinase. Curr Biol. 1995;5:296-305. [DOI] |
| 21. | Yokohari K, Yamashita Y, Okada S, Ohya K, Oda S, Hatano M, Mano H, Hirasawa H, Tokuhisa T. Isoform-dependent interaction of BRDG1 with Tec kinase. Biochem Biophys Res Commun. 2001;289:414-420. [PubMed] [DOI] |
| 22. | Altman A, Kaminski S, Busuttil V, Droin N, Hu J, Tadevosyan Y, Hipskind RA, Villalba M. Positive feedback regulation of PLCgamma1/Ca(2+) signaling by PKCtheta in restimulated T cells via a Tec kinase-dependent pathway. Eur J Immunol. 2004;34:2001-2011. [PubMed] [DOI] |
| 23. | Schoen JM, Lautt WW. Nitric oxide potentiates C-Fos mRNA expression after 2/3 partial hepatectomy. Proc West Pharmacol Soc. 2002;45:47-48. [PubMed] |
| 24. | Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477-1487. [PubMed] [DOI] |
| 25. | Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277:28411-28417. [PubMed] [DOI] |
| 26. | Debonera F, Aldeguer X, Shen X, Gelman AE, Gao F, Que X, Greenbaum LE, Furth EE, Taub R, Olthoff KM. Activation of interleukin-6/STAT3 and liver regeneration following transplantation. J Surg Res. 2001;96:289-295. [PubMed] [DOI] |
| 27. | Alonzi T, Maritano D, Gorgoni B, Rizzuto G, Libert C, Poli V. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol Cell Biol. 2001;21:1621-1632. [PubMed] [DOI] |
| 28. | Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA. 2004;101:4477-4482. [PubMed] [DOI] |
| 29. | Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA. 2004;101:10608-10613. [PubMed] [DOI] |
| 30. | Okano J, Shiota G, Matsumoto K, Yasui S, Kurimasa A, Hisatome I, Steinberg P, Murawaki Y. Hepatocyte growth factor exerts a proliferative effect on oval cells through the PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2003;309:298-304. [PubMed] [DOI] |