修回日期: 2004-11-20
接受日期: 2004-11-27
在线出版日期: 2004-12-15
目的: 研究缺氧诱导因子1-α(HIF-1α)在大肠肿瘤中的表达及其与凋亡、增生的关系, 探讨HIF-1α在大肠癌发生发展中的作用.
方法: 运用免疫组化的方法检测HIF-1α, Bcl-2, Bax, PCNA在正常大肠组织13例, 大肠腺瘤26例, 大肠癌50例中的表达.
结果: 正常大肠组织HIF-1α均为阴性表达, 腺瘤及癌组织中HIF-1α阳性表达率为30.8%和64%, 大肠癌HIF-1α表达率显著高于腺瘤(χ2 = 8.546, P<0.01). 大肠癌HIF-1α表达与浸润, 淋巴结转移, Dukes分期相关(χ2 = 6.339, P<0.05; χ2 = 9.091, P<0.01; χ2 = 10.72, P<0.05). HIF-1α表达与肿瘤大小、分化程度无关(P>0.05). Bcl-2在3组中阳性表达率为15.4%, 50.0%和76.0%, 三者间表达率差别有显著意义(P<0.05). Bax在3组中阳性表达率为76.9%, 65.4%和58.0%, 三者间表达率无显著性差异(P>0.05). Bcl-2, Bax表达与肿瘤大小, 分化, Dukes分期无关(P>0.05). 正常大肠组织PCNA表达均为低增生活性, 腺瘤及癌中PCNA高增生活性者26.9%和56.0%, 癌组织PCNA高增生活性表达显著高于腺瘤(χ2 = 5.073, P<0.05). 大肠癌增生程度与浸润及Dukes分期相关(χ2 = 6.336, P<0.05; χ2 = 11.219, P<0.01). 腺瘤及大肠癌HIF-1α表达与Bcl-2, PCNA增生程度呈正相关(r = 0.5, r = 0.535, P<0.05; r = 0.457, r = 0.426, P<0.01), 与Bax表达无关.
结论: HIF-1α抑制大肠肿瘤凋亡, 促进增生, 与肿瘤浸润转移密切相关, 在大肠癌发生发展中发挥重要作用.
引文著录: 程胜平, 刘南植, 钱颉. 大肠腺瘤及癌组织HIF-1α的表达与凋亡增生的关系. 世界华人消化杂志 2004; 12(12): 2792-2796
Revised: November 20, 2004
Accepted: November 27, 2004
Published online: December 15, 2004
AIM: To study the expression of HIF-1α and its relationship with apoptosis and proliferation in human colorectal neoplasm
METHODS: Expression of HIF-1α, Bcl-2, Bax and PCNA was detected in normal colorectal tissue (n = 13), colorectal adenoma (n = 26) and adenocarcinoma (n = 50) by the immunohistochemical method respectively.
RESULTS: HIF-1α was negatively expressed in normal colorectal mucosa tissues. The positive rate of HIF-1α expression was significantly higher in adenoma tissues than that in adenocarcinoma ones (30.8% vs 64.0%, χ2 = 8.546, P < 0.05). The expression of HIF-1α in colorectal carcinoma significantly related to the depth of invasion, lymph node metastasis, and Dukes staging (χ2 = 6.339, P < 0.05; χ2 = 9.091, P < 0.01; χ2 = 10.72, P < 0.05). No significant correlation was found between the positive rate of HIF-1α and tumor's size and differentiation (P > 0.05). The positive rate of Bcl-2 expression in three groups was 15.4%, 50.0% and 76.0% respectively, and there were significant differences among them (P < 0.05). The positive rate of Bax expression was 76.9%, 65.4% and 58.0% in three groups respectively. There were no significant differences among them. The proliferation level of carcinoma is higher than that of adenoma (χ2 = 5.073, P < 0.05) and it was related to the depth of invasion and Dukes staging (χ2 = 6.336, P < 0.05; χ2 = 11.219, P < 0.01). HIF-1α expression was positively associated with Bcl-2 and PCNA levels in adenoma and carcinoma (r = 0.5, r = 0.535, P < 0.05; r = 0.457, r = 0.426, P < 0.01).
CONCLUSION: Over-expression of HIF-1α is associated with apoptosis inhibition, proliferation, invasion and metastasis of colorectal neoplasm, and it may play an important role in the caicinogenesis and development of colorectal carcinoma.
- Citation: Cheng SP, Liu NZ, Qian J. Expression of HIF-1α and its relations with apoptosis and proliferation in colorectal neoplasm. Shijie Huaren Xiaohua Zazhi 2004; 12(12): 2792-2796
- URL: https://www.wjgnet.com/1009-3079/full/v12/i12/2792.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i12.2792
缺氧诱导因子-1(HIF-1)是迄今为止发现惟一的低氧状态下发挥活性的转录因子. HIF-1是由HIF-1α及HIF-1α组成的异二聚体, 其中α亚单位被认为是特异性氧调节亚单位, 决定了HIF-1的活性. 人类实体肿瘤中常存在缺氧环境, 已在多种恶性肿瘤甚至癌前病变中检测到HIF-1α过表达[1-7]. HIF-1α参加了肿瘤对低氧的适应过程, 从而增强了肿瘤细胞对缺氧的抵抗能力, 促进肿瘤细胞的生长及恶性转化[8-12]. 研究表明, HIF-1α在缺氧介导的凋亡及增生中起重要作用, 但目前关于HIF-1α与凋亡、增生的关系报道不一[13-15]. 大肠癌是常见的恶性肿瘤, 研究认为凋亡及增生的失衡在大肠黏膜癌变中起着重要作用. 关于HIF-1α与大肠肿瘤凋亡及增生的研究较少. 我们通过免疫组化的方法检测HIF-1α, Bcl-2, Bax, PCNA在正常大肠黏膜, 腺瘤及腺癌中的表达, 探讨大肠肿瘤临床病理及HIF-1α与凋亡及增生的关系.
2000/2003年大肠腺癌50例, 男28例, 女22例, 年龄34-67(52.9±10.6岁). 高分化17例, 中分化24例, 低分化9例. Dukes A 11例, B 11例, C 18例, D 10例. 大肠腺瘤26例, 管状腺瘤10例, 绒毛状腺瘤9例, 管状-绒毛状腺瘤7例. 另13例正常大肠组织作对照. 石蜡切片厚5 μm. 兔抗人HIF-1α多抗(1:80)及SABC试剂盒(购自武汉博士德生物工程有限公司), 鼠抗人Bcl-2, Bax, PCNA单抗(1:100, 北京中山试剂公司).
采用免疫组化方法检测. HIF-1α免疫组化染色采用SABC法, Bcl-2, Bax, PCNA采用SP法. 染色步骤按试剂盒说明进行, 染色前用柠檬酸抗原修复液热水浴或微波抗原修复10 min. 用PBS代替一抗作阴性对照, 已知阳性切片作阳性对照. HIF-1α蛋白以胞质或胞核内有棕黄色颗粒为阳性, 高倍镜下(×400)对每张切片随机选择5个视野, 计数200个细胞/视野, 共计1 000个, 阳性细胞数<1%为阴性(-), 阳性细胞数1-10%为弱阳性(+), 11-50%为中度阳性(++), >51%为强阳性(+++)[13]. Bcl-2和Bax蛋白均以胞质内有棕黄色颗粒为阳性. 判定标准: 无阳性反应为阴性(-), 阳性细胞数<5%为弱阳性(+), 6-50%为中度阳性(++), >51%为强阳性(+++). PCNA以胞核内有棕黄色颗粒为阳性, <5%为阴性, 6-25%为(+), 26-50%(++), 51-75%(+++), >76%(++++), 以+++, ++++级为高增生活性, 以此作为大肠肿瘤的增生程度指标.
统计学处理 根据数据性质, 采用χ2检验, Fishers精确概率法, 及Spearman等级相关分析, 所有数据在SPSS12.0软件进行, 显著水平取α = 0.05.
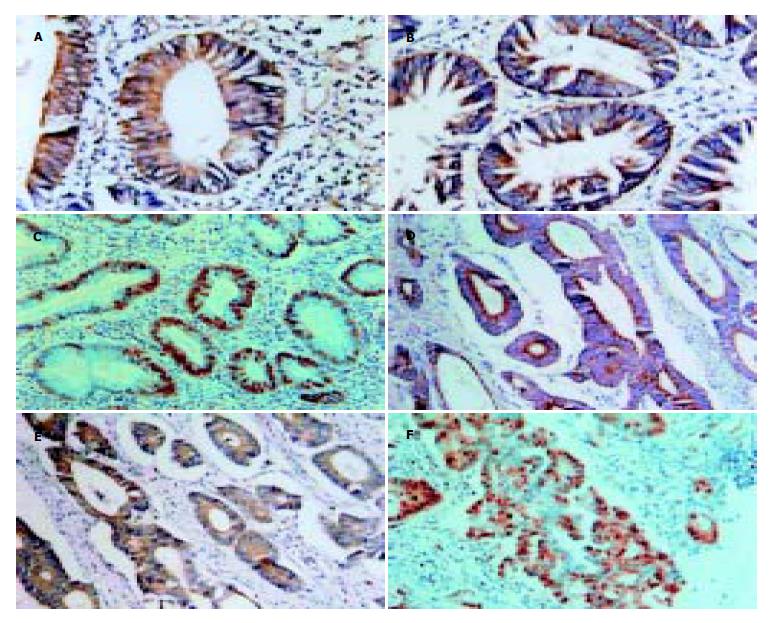
HIF-1α蛋白主要表达于胞质, 也有部分胞核表达. 正常大肠组织HIF-1α均为阴性表达, 腺瘤中阳性表达率为30.8%(8/26), 癌组织中阳性表达率为64.0%(32/50). 腺瘤中HIF-1α阳性表达率高于正常组织(χ2 = 7.483, P<0.05), 腺癌HIF-1α阳性表达率显著高于腺瘤(χ2 = 8.546, P<0.01). Bcl-2及Bax表达于胞质. Bcl-2在正常组织, 腺瘤, 癌组织中阳性表达率为15.4%, 50.0%, 76.0%, Bcl-2的阳性表达率正常组织<腺瘤<癌组织, 三者之间表达率差别有显著意义(P<0.05). Bax在三者中阳性表达率为76.9%, 65.4%, 58.0%, 呈逐渐下降趋势, 但经统计分析, 三者间表达率无显著性差异(P>0.05). PCNA表达于细胞核. 正常大肠组织PCNA表达均为低增生活性, 腺瘤中PCNA高增生活性者26.9%(7/26), 癌组织中高增生活性56.0%(28/50). 腺瘤中高增生活性表达率虽高于正常组织, 但其差异无显著性(P = 0.07, Fishers精确概率), 癌组织中PCNA高增生活性表达显著高于腺瘤(χ2 = 5.073, P<0.05, 表1, 图1A-F).
| 分组 | n | HIF-1α | Bcl-2 | Bax | PCNA | ||||
| - | + | - | + | - | + | 低 | 高 | ||
| 正常 | 13 | 13 | 0 | 11 | 2(15.4) | 3 | 10(76.9) | 13 | 0 |
| 腺瘤 | 26 | 18 | 8(30.8) | 13 | 13(50.0) | 9 | 17(65.4) | 19 | 7(26.9) |
| 癌组织 | 50 | 18 | 32(64.0) | 12 | 38(76.0) | 21 | 29(58.0) | 22 | 28(56.0) |
HIF-1α阳性表达率与大肠癌的大小, 分化程度无关(P>0.05). 浸润浆膜及有淋巴结转移者HIF-1α阳性表达率高于未浸润浆膜(χ2 = 6.339, P<0.05)及无淋巴结转移者(χ2 = 9.091, P<0.01), 随着Dukes分期进展, HIF-1α表达增高, 各期HIF-1α表达率有显著性差异(χ2 = 10.72, P<0.05). PCNA增生程度与肿瘤的大小及分化程度无关(P>0.05), 浸润浆膜者增生程度高于未浸润浆膜者(χ2 = 6.336, P<0.05), 有淋巴结转移者增生活性高于无淋巴结转移者, 其差异处于统计学临界范围(χ2 = 3.63, P = 0.057). 随着Dukes分期进展, PCNA增生程度增高, 各期PCNA增生程度差异具显著性(χ2 = 11.219, P<0.01). Bcl-2, Bax在大肠癌中的表达与肿瘤的大小、分化及Dukes分期无明显相关(P>0.05, 表2).
在大肠腺瘤中, HIF-1α表达与Bcl-2, PCNA增生程度呈正相关(r = 0.5, r = 0.535, P<0.05), 与Bax表达无关(r = 0.135, P>0.05). 在大肠癌中, HIF-1α表达与Bcl-2表达呈正相关(r = 0.457, χ2 = 8.315, P<0.01), 与PCNA增生程度呈正相关(r = 0.426, χ2 = 9.091, P<0.01), 与Bax表达无关(r = -0.132, χ2 = 0.867, P>0.05, 表3).
缺氧是实体肿瘤常见现象, 能诱导一系列与血管新生, 无氧代谢等密切相关的基因, 帮助肿瘤细胞适应缺氧环境. 在此过程中起关键作用的是HIF-1α. HIF-1α过表达与肿瘤的浸润转移、预后、治疗抵抗密切相关[16-19]. 本结果显示, HIF-1α在正常大肠组织中无表达, 在腺瘤中阳性表达率为30.8%, 在癌组织中表达率为64.0%, 三者之间表达率差异有显著性. HIF-1α表达水平在正常组织-腺瘤-腺癌中不断增高, 这与江从庆et al[20], Jiang et al[21]结果一致. 这表明细胞缺氧出现在癌变之前并持续到肿瘤发展的全过程, HIF-1α过表达可能是癌变过程中的早期行为, 提示HIF-1α过度表达在促进腺瘤癌变中发挥重要作用. 我们还发现, 浸润浆膜, 有淋巴结转移者HIF-1α阳性表达率高于未浸润浆膜及无淋巴结转移者, 且随着Dukes分期进展, HIF-1α表达率逐渐增高, 表明HIF-1α在大肠癌的浸润, 转移中起重要作用.
Bcl-2是公认的抗凋亡基因, Bax基因通过拮抗Bcl-2基因而发挥其促进细胞凋亡的作用, Bcl-2/Bax比值与细胞凋亡率显著相关. Bedi et al[22]研究表明在大肠黏膜癌变过程中细胞凋亡能力逐渐减弱. 於亮亮et al[23]研究, 在结肠癌变过程中, 从腺瘤病变到癌, Bcl-2表达逐渐增高, 凋亡指数相应降低. 说明Bcl-2异常表达是大肠癌变过程细胞凋亡受抑制的潜在机制. 我们也发现, 在正常大肠组织, 腺瘤, 癌组织中Bcl-2表达逐渐增高, 三组表达有显著性差异, 而Bax表达有逐渐降低趋势, 表明在大肠腺瘤-癌变序列中, 细胞凋亡受抑制. PCNA是DNA聚合酶的辅酶之一, 可作为细胞周期内S期的特异性标志和评价细胞增生状态的一个指标[24]. 本结果显示, 腺瘤细胞增生活性较正常黏膜增加, 而癌组织又较腺瘤明显增加, 从腺瘤到癌发展过程中, 细胞增生持续增加.
HIF-1α在低氧诱导的凋亡与肿瘤增生中起重要作用. 但关于缺氧及HIF-1α是促进或抑制凋亡目前报道不一[25-27]. 目前关于HIF-1α与大肠肿瘤凋亡关系报道较少. 我们观察了HIF-1α在大肠腺瘤及癌中的表达及其与Bcl-2, Bax表达的关系, 结果显示HIF-1α, Bcl-2表达从腺瘤到癌逐渐增多, 且HIF-1α表达在腺瘤及癌中均与Bcl-2呈正相关, 与Bax表达无关. 这提示HIF-1α表达与大肠肿瘤凋亡抑制有关, 在大肠腺瘤-癌演变过程中, HIF-1α通过抑制细胞凋亡从而促进大肠腺瘤的恶性转化过程. 大肠肿瘤中缺氧及HIF-1α调控凋亡机制尚不明确. Kinoshita et al[28]在人结肠癌细胞系HCT116培养中发现, 缺氧培养24 h后, 肿瘤细胞Bcl-2表达上调, 持续缺氧培养48 h后, Bcl-2表达较前更加增高, 且同时伴有PCNA高表达. 认为缺氧可上调Bcl-2表达从而使肿瘤细胞抵御缺氧所致的凋亡而继续生长. Wang et al[29]也报道, 缺氧培养的HTDEC细胞(human tumor-derived endothelial cells, 分离自结肠癌)Bcl-2表达上调从而使HTDEC细胞抵抗IFN-α诱导的凋亡, 并认为缺氧上调Bcl-2是通过HIF-1α介导. 有研究表明, VEGF表达上调可以促进Bcl-2表达从而抵御缺氧所致的凋亡[30-32]. VEGF是HIF-1α最重要的调控基因, 研究已表明在大肠肿瘤中HIF-1α诱导VEGF表达上调. 可见HIF-1α可能与其他基因一起参与对凋亡的调控. 樊利芳et al[13]发现在肺癌中HIF-1α与bcl-2呈负相关, 而与bax正相关, 与本研究不一致, 可能与不同的肿瘤组织类型有关.
本实验结果显示PCNA增生活性在正常黏膜-腺瘤-癌中逐渐增高, 且PCNA高增生活性与大肠癌浸润, Dukes分期有关. 这与陈卫昌et al[33]研究结果一致. 提示PCNA可作为判断大肠癌浸润转移的指标. Kinoshita et al[28]在大肠癌HCT116细胞系缺氧培养发现PCNA高表达. 本实验结果也显示HIF-1α表达与腺瘤及癌组织PCNA 增生活性呈正相关, 提示HIF-1α过表达可促进肿瘤组织增生. HIF-1α促进肿瘤细胞增生可能与HIF-1α促进VEGF, IGF-1, IGF-2等基因表达有关.
总之, 大肠肿瘤组织局部缺氧, HIF-1α表达上调, 诱导其下游基因表达, 使肿瘤细胞适应缺氧环境, 反过来又抑制缺氧所致的凋亡及促进增生, 使凋亡/增生进一步失衡, 从而最终导致癌变发生发展.
编辑: N/A
| 1. | Birner P, Schindl M, Obermair A, Breitenecker G, Oberhuber G. Expression of hypoxia-inducible factor 1alpha in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res. 2001;7:1661-1668. [PubMed] |
| 3. | Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst. 2001;93:309-314. [PubMed] [DOI] |
| 4. | Birner P, Gatterbauer B, Oberhuber G, Schindl M, Rossler K, Prodinger A, Budka H, Hainfellner JA. Expression of hypoxia-inducible factor-1? in oligodendrogliomas: Its impact on prognosis and on neoangiogenesis. Cancer. 2001;92:165-171. [DOI] |
| 5. | Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, Ito M, Chayama K. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105:176-181. [PubMed] [DOI] |
| 6. | Shibaji T, Nagao M, Ikeda N, Kanehiro H, Hisanaga M, Ko S, Fukumoto A, Nakajima Y. Prognostic significance of HIF-1 alpha overexpression in human pancreatic cancer. Anticancer Res. 2003;23:4721-4727. [PubMed] |
| 7. | Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P, Oberhuber G; Austrian Breast and Colorectal Cancer Study Group. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res. 2002;8:1831-1837. [PubMed] |
| 8. | Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62-S67. [PubMed] [DOI] |
| 9. | Semenza GL. Involvement of hypoxia-inducible factor 1 in human cancer. Intern Med. 2002;41:79-83. [PubMed] [DOI] |
| 10. | Choi KS, Bae MK, Jeong JW, Moon HE, Kim KW. Hypoxia-induced angiogenesis during carcinogenesis. J Biochem Mol Biol. 2003;36:120-127. [DOI] |
| 12. | Acker T, Plate KH. A role for hypoxia and hypoxia-inducible transcription factors in tumor physiology. J Mol Med (Berl). 2002;80:562-575. [PubMed] [DOI] |
| 13. | 樊 利芳, 刁 路明, 陈 德基, 刘 铭球, 朱 丽琴, 李 红钢, 唐 志佼, 夏 东, 刘 绚, 陈 洪雷. 肺癌组织中缺氧诱导因子-1?的表达及其与凋亡和增生的关系. 癌症. 2002;21:254-258. |
| 14. | Erler JT, Cawthorne CJ, Williams KJ, Koritzinsky M, Wouters BG, Wilson C, Miller C, Demonacos C, Stratford IJ, Dive C. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004;24:2875-2889. [PubMed] [DOI] |
| 15. | Goda N, Dozier SJ, Johnson RS. HIF-1 in cell cycle regulation, apoptosis, and tumor progression. Antioxid Redox Signal. 2003;5:467-473. [PubMed] [DOI] |
| 16. | Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297-307. [DOI] |
| 17. | Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, Semenza GL. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911-2916. [PubMed] |
| 18. | Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693-4696. [PubMed] |
| 19. | Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138-1143. [PubMed] |
| 21. | Jiang YA, Fan LF, Jiang CQ, Zhang YY, Luo HS, Tang ZJ, Xia D, Wang M. Expression and significance of PTEN, hypoxia-inducible factor-1 alpha in colorectal adenoma and adenocarcinoma. World J Gastroenterol. 2003;9:491-494. [PubMed] [DOI] |
| 22. | Bedi A, Pasricha PJ, Akhtar AJ, Barber JP, Bedi GC, Giardiello FM, Zehnbauer BA, Hamilton SR, Jones RJ. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811-1816. [PubMed] |
| 24. | Yue SQ, Yang YL, Dou KF, Li KZ. Expression of PCNA and CD44mRNA in colorectal cancer with venous invasion and its relationship to liver metastasis. World J Gastroenterol. 2003;9:2863-2865. [PubMed] [DOI] |
| 25. | Piret JP, Mottet D, Raes M, Michiels C. Is HIF-1alpha a pro- or an anti-apoptotic protein? Biochem Pharmaco. 2002;64:889-892. [DOI] |
| 26. | Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura Ki, Hosokawa M, Asaka M. Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001;61:6548-6554. [PubMed] |
| 27. | Piret JP, Lecocq C, Toffoli S, Ninane N, Raes M, Michiels C. Hypoxia and CoCl2 protect HepG2 cells against serum deprivation- and t-BHP-induced apoptosis: a possible anti-apoptotic role for HIF-1. Exp Cell Res. 2004;295:340-349. [PubMed] [DOI] |
| 28. | Kinoshita M, Johnson DL, Shatney CH, Lee YL, Mochizuki H. Cancer cells surviving hypoxia obtain hypoxia resistance and maintain anti-apoptotic potential under reoxygenation. Int J Cancer. 2001;91:322-326. [DOI] |
| 29. | Wang JH, Wu QD, Bouchier-Hayes D, Redmond HP. Hypoxia upregulates Bcl-2 expression and suppresses interferon-gamma induced antiangiogenic activity in human tumor derived endothelial cells. Cancer. 2002;94:2745-2755. [PubMed] [DOI] |
| 30. | Iervolino A, Trisciuoglio D, Ribatti D, Candiloro A, Biroccio A, Zupi G, Del Bufalo D. Bcl-2 overexpression in human melanoma cells increases angiogenesis through VEGF mRNA stabilization and HIF-1-mediated transcriptional activity. FASEB J. 2002;16:1453-1455. [PubMed] |
| 31. | Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375-384. [PubMed] [DOI] |