修回日期: 2004-08-10
接受日期: 2004-08-21
在线出版日期: 2004-11-15
目的: 探讨胃癌及癌旁组织中H pylori感染和PTEN、cyclinE表达, 他们之间的相关性以及胃癌发生的可能机制.
方法: 每例标本采用快速尿素酶试验, 组织病理学检测两种方法检查H pylori, 采用免疫组化法检测胃癌组织及癌旁组织59例中PTEN及cyclinE的表达.
结果: 胃癌组织PTEN在阳性率明显低于癌旁组织, 二者之间有显著差异(50.85% vs 96.61% P<0.05); 胃癌组织cyclinE阳性率高于癌旁组织, 二者之间有显著差异(55.93% vs 40.7% P>0.05); PTEN在高中分化腺癌中的表达率明显高于低分化腺癌和黏液癌, 有显著差异(68.4% vs 33.3% P<0.05, 68.4% vs 37.5%, P<0.05); 胃黏液癌及低分化腺癌CyclinE阳性表达明显高于高中分化腺癌, 有显著差异(68.8% vs 31.5% P<0.05, 66.7% vs 31.5%, P<0.05); H pylori阳性的PTEN阳性表达率明显低于H pylori阴性, 二者之间有显著差异(24.2% vs 69.2% P<0.05); cyclinE表达阳性率在H pylori阳性和阴性胃癌中, 二者之间无显著差异(51.9% vs 59.4% P>0.05).
结论: 胃癌的发生与抑癌基因PTEN的下调和癌基因cyclinE的过度表达有关, H pylori感染的致癌机制中可能有抑癌基因PTEN参与.
引文著录: 周庆华, 刘丽娜, 吕申, 王梅, 刘春英. 胃癌组织PTEN、cyclinE表达与幽门螺旋杆菌感染的关系. 世界华人消化杂志 2004; 12(11): 2560-2563
Revised: August 10, 2004
Accepted: August 21, 2004
Published online: November 15, 2004
AIM: To investigate the relationship between H pylori infection and PTEN, cyclinE expression in gastric cancer and adjacent mucosal tissues, and to study the possible mechanism of H pylori in gastric carcinogenesis.
METHODS: Both rapid urease and pathological test were used to examine H pylori in 59 cases of specimen. The expression of PTEN and cyclinE in gastric cancer and adjacent mucosal tissues was detected by immunohistochemical technique (SP method).
RESULTS: PTEN expression in gastric cancer tissues was significantly lower than that in the cancer adjacent tissues (50.85% vs 96.61%, P < 0.05); however, CyclinE expression in gastric cancer tissues was significantly higher than that in cancer adjacent tissues. The level of PTEN expression in well-differentiated adenocarcinoma was significantly higher than that in poorly-differentiated adenocarcinoma and mucinous carcinoma (68.4% vs 33.3%, P < 0.05 68.4% vs 37.5%, P < 0.05 respectively); however, the level of CyclinE expression in well-differentiated adenocarcinoma was significantly lower than that in poorly-differentiated adenocarcinoma and mucinous carcinoma (31.5% vs 68.8%, P < 0.05; 31.5% vs 66.7%, P < 0.05); PTEN expression in in H pylori positive group was lower than that in H pylori negative group (51.9% vs 59.4%, P > 0.05); CyclinE expression had no significant difference between H pylori positive and negative gastric cancer tissues.
CONCLUSION: Pathogenesis of gastric cancer relates to loss of suppression gene PTEN and over-expression of oncogene cyclinE, and suppression gene PTEN probably plays a role in development of gastric cancer induced by H pylori infection.
- Citation: Zhou QH, Liu LN, Lv S, Wang M, Liu CY. Relationship between expression of PTEN and cyclinE and Helicobacter pylori infection in gastric cancer. Shijie Huaren Xiaohua Zazhi 2004; 12(11): 2560-2563
- URL: https://www.wjgnet.com/1009-3079/full/v12/i11/2560.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i11.2560
PTEN/MMAC1/TEP1即细胞骨架蛋白同源的磷酸酶的编码蛋白(phosphatase homologue PTEN)[1], 是目前发现的第一个具有磷酸酯酶活性的抑癌基因, 既有抑制生长作用, 又有促进细胞凋亡作用, 参与细胞周期以及抑制细胞黏附及肿瘤转移[2]. 而癌基因cyclinE是细胞进入S期的关键性调节基因, 加速G1-S期转化, 在G1晚期起作用[3], 与人类肿瘤有关[4]. H pylori与胃癌发生有密切关系, 但引起胃癌的确切机制目前尚不明确, 有学者认为与肿瘤相关基因的异常表达有关[5-6].
2003-01/2003-10大连医科大学附属一院经胃镜检查诊断为胃癌, 并于镜下活检以及外科行胃大部切除的标本59例, 均经病理确诊并分型, 其中高分化腺癌为19例, 低分化腺癌24 例, 黏液腺癌16例. 在胃镜下取得的癌旁组织距癌组织至少5 cm, 在胃大部切除术后取得的癌旁组织为与癌组织最远处, 也经病理确诊并分型, 其中肠化生26例, 胃炎34例, 所有患者受检前均未经放化疗等治疗. 患者禁食10 h后于胃镜直视下取标本, 胃癌组织3块, 并经快速尿素酶试验检查, 以确定有无H pylori感染, 之后再用于组织学检查. 男43例, 女16例, 平均年龄为62.1±25.6岁, 所有标本经40 g/L中性甲醛固定, 石蜡包埋, 连续切片厚3-5μm. 常规HE染色, 根据组织结构和细胞形态诊断肠化生组织、胃炎组织、高中分化腺癌、低分化腺癌、胃黏液癌.
H pylori检测: 采用尿素酶试验法及组织学诊断, 后者用改良Giemsa染色法, 二种方法均阳性定为H pylori阳性;二者均阴性定为H pylori 阴性. 免疫组化染色: PTEN、cyclinE抗体分别设阴性和阳性对照, 阳性对照为已知表达阳性的组织, 阴性对照以PBS代替一抗. 免疫组化所用鼠抗人mAbPTEN、cyclinE及SP、DAB试剂均购于北京中山生物技术有限公司. 操作步骤按说明进行.免疫组化染色结果的判定: 检测PTEN、cyclinE阳性信号在细胞核内呈现棕褐色颗粒, 以阳性细胞的百分比判断结果. 每例切片选5个区域40倍高倍视野, 每个区域记数200个细胞, 按阳性细胞占同类计数细胞的百分比将免疫组化结果分为阴性, 即百分比<5%; 阳性为百分比>5%. 实验结果拍摄成像.
统计学处理 采用SPSS软件进行统计分析, 采用χ2检验对免疫组化结果进行分析处理, 以P<0.05(双侧)为有统计学意义.
PTEN、和cyclinE的表达在59例胃癌组织中有30例PTEN阳性, 阳性率50.9%. 59例癌旁组织中有57例PTEN阳性, 阳性率为96.6%, 二者之间有显著性差异(χ2 = 31.89, P<0.05, 表1). 59例胃癌组织中有33例cyclinE阳性, 阳性率为55.9%, 在癌旁组织中有24例阳性, 阳性率为40.7%, 二者之间无显著性差(χ2 = 2.74, P>0.05).
| 组织 | PTEN | CyclinE | ||||
| + | - | 阳性率(%) | + | - | 阳性率(%) | |
| 胃癌 | 30 | 29 | 50.9 | 33 | 26 | 55.9 |
| 癌旁 | 57 | 2 | 96.6 | 24 | 35 | 40.7 |
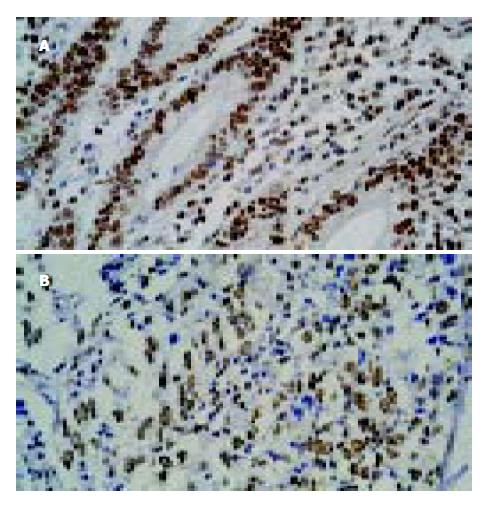
2.2 19例高中分化腺癌中, 13例PTEN阳性(图1), 阳性率为68.4%, 24例低分化腺癌中8例PTEN阳性, 阳性率为33.3%, 在16例黏液癌中有6例阳性, 阳性率为37.5%, 高中分化腺癌中的表达率明显高于低分化腺癌和黏液癌, 与二者比较有显著性差异(分别为χ2 = 5.58 P<0.05; χ2 = 6.3, P<0.05). 黏液癌与低分化腺之间无显著性差异(χ2 = 0.11, P>0.05). PTEN在癌旁组织中, 25例肠化生组织中有23例阳性, 阳性率为92.0%, 而在34例胃炎组织全部阳性, 阳性率为100%, 肠化生组织的阳性表达率虽低于胃炎组织, 但二者之间无显著性差异(χ2 = 0.16, P>0.05). CyclinE在胃黏液癌、胃低分化腺癌、胃高中分化腺癌中阳性表达分别为11、16、6例; cyclinE阳性表达率分别为68.8%、66.7%及31.6%, 胃黏液癌阳性表达明显高于胃高中分化腺癌, 有显著性差异(χ2 = 4.11, P<0.05), 胃低分化腺癌阳性表达也明显高于胃高中分化腺癌, 有显著性差异(χ2 = 5.23, P<0.05); 胃黏液癌阳性表达略高于胃低分化腺癌, 二者之间无显著性差异(χ2 = 0.045, P>0.05). 癌旁组织中cyclinE在25例肠化生组织中的14例阳性, 阳性率56.0%, 34例胃炎组织中, cyclinE有9例阳性, 阳性表达率为26.5%, 肠化生组织中cyclinE的阳性表达率明显高于胃炎组织, 二者之间有显著性差异(χ2 = 9.47, P<0.05, 表2).
| 病理 | n | PTEN | CyclinE | ||||
| + | - | 阳性率(%) | + | - | 阳性率(%) | ||
| 肠化生 | 25 | 23 | 2 | 92.0 | 14 | 11 | 56.0 |
| 胃炎组织 | 34 | 34 | 0 | 100 | 9 | 25 | 26.5 |
| 高中分化腺癌 | 19 | 14 | 5 | 73.7 | 6 | 13 | 31.6 |
| 低分化腺癌 | 24 | 9 | 15 | 37.5 | 16 | 8 | 66.7 |
| 黏液癌 | 16 | 5 | 11 | 31.3 | 11 | 5 | 68.8 |
| 总计 | 118 | 30 | 88 | 56 | 62 | ||
在H pylori阳性和H pylori阴性胃癌中, PTEN表达阳性率分别为36.3%和76.5%(表3), H pylori阳性者明显低于H pylori阴性, 二者之间有显著性差异(χ2 = 11.94, P<0.05). 在H pylori阳性和H pylori阴性胃癌中, cyclinE表达阳性率分别为51.9%及59.4%, 二者之间无显著差异(χ2 = 0.34, P>0.05).
| H pylori | PTEN | CyclinE | ||||
| + | - | 阳性率(%) | + | - | 阳性率(%) | |
| + | 8 | 25 | 24.2 | 14 | 13 | 51.9 |
| - | 18 | 8 | 69.2 | 19 | 13 | 59.4 |
| 26 | 33 | 33 | 26 | |||
文献报道, PTEN作为一种抑癌基因, 参与细胞凋亡调控[7-8]. 近年研究发现, 其表达与一些恶性肿瘤发生发展、生物学行为和预后有密切的关系[9-10], 但对PTEN在胃癌中的表达却有不一致的报道[11-12]. 本结果显示, PTEN在癌组织中的表达明显低于癌旁组织, 提示在胃癌组织中PTEN表达下调, 在由胃炎组织或肠化生组织向癌组织进化演变中抑癌基因PTEN的减少会导致对肿瘤的抑制作用的减弱. 本资料还显示, PTEN在高中分化腺癌阳性表达明显低于低分化腺癌及黏液腺癌, 而在低分化腺癌和黏液癌中的表达无明显差别, 提示PTEN表达与胃癌的临床病理特征及生物学行为有密切关系. PTEN阳性表达越低其肿瘤的恶性程度越高, 愈后越差. 此结果与有关报道一致[13-15]. CyclinE与细胞周期素依赖激酶2(CDK2)结合形成复合物, 该复合物的浓度是细胞通过G1-S调解点的最关键因素[16-18]. 异常细胞内cyclinE无顺序、无计划地过渡表达, 在整个细胞周期中含量过高, 可持续地激活CDK2区磷酸化底物, 促进细胞由G1期进入S期, 驱使细胞超越某些控制机制异常增生[19-20]. CyclinE过度表达与细胞的肿瘤明显相关[21-22]. 我们发现在高分化腺癌, 低分化腺癌及黏液腺癌中cyclinE的表达阳性率分别为31.6%、66.7%及68.8%, 说明随着肿瘤恶性程度升高、分化程度降低, cyclinE的表达率增高, 提示cyclinE与细胞分化和肿瘤的恶性程度有关. 结果还发现cyclinE的表达在癌组织高于癌旁组织, 提示癌基因cyclinE参与胃癌的发生. 在癌旁组织中, 肠化生组织的表达高于胃炎组织. 肠化生组织可视为一个癌前病变, 在肠化生组织中cyclinE增高提示在胃癌发生早期有cyclinE的参与. 关于肠化生组织的cyclinE阳性表达高于高分化腺癌, 考虑原因如下; (1)低分化腺癌及黏液腺癌的癌旁组织中, 有肠化生的为22例, 占所有肠化生病例(25例)的88%为大多数; (2)CyclinE的表达率与癌的恶性程度相关, 且多发生在癌的早期, 故在低分化腺癌及黏液腺癌癌旁组织中, cyclinE的表达率较高为60.4%, 以上两个原因使总的肠化生组织中的阳性表达率较高为56.0%, 而高分化腺癌恶性程度低, 其癌旁组织中肠化生的例数少, 为3例, 仅占12%, 对总的肠化生中的cyclinE的表达率影响小, 故肠化生的cyclinE的阳性表达率高于高分化腺癌的31.6%.
幽门螺杆菌(H pylori )是重要的致癌因素已受到越来越多的资料的证实[23-24]. 当幽门螺杆菌感染后, 宿主细胞对H pylori产生的反应分为速发性和迟发性两类, 迟发性反应多数涉及到基因表达的变化[25]. 近期一些研究者应用cDNA array分析了H pylori诱导的胃上皮细胞基因表达谱的变化. Chiou et al报道, 在H pylori的刺激下, 胃癌细胞株有21个基因上调和17个基因下调[26]. 所以对基因表达变化的进一步研究, 将有助于了解宿主细胞的抵抗策略和适应性变化的根本机制, 从而为干预有关环节, 减轻宿主细胞对H pylori的易感性提供新的思路[27-28]. 我们发现cyclinE在H pylori阳性的组织中与H pylori阴性组织中分别为51.9%及59.4%二者无显著性差异, 提示cyclinE在胃癌中的表达变化可能与H pylori感染无明显相关性. PTEN在H pylori阳性患者中为36.3%, 低于在阴性患者中的76.9%, 有显著性差异, 提示H pylori感染的胃癌患者中可能有PTEN的表达下调, 二者之间可能有一定的相关性. 关于cyclinE、PTEN与H pylori感染的关系, 文献报道甚少, 其他抑癌基因和癌基因与H pylori在胃癌的关系研究亦鲜有报道. 因此, H pylori感染导致胃癌的途径有很多种[29-30], 能否通过对癌基因及抑癌基因的影响而致癌, 还需进一步研究.
编辑: N/A
| 1. | Lee C, Kim JS, Waldman T. PTEN gene targeting reveals a radiation-induced size checkpoint in human cancer cells. Cancer Res. 2004;64:6906-6914. [PubMed] [DOI] |
| 4. | Yu J, Miehlke S, Ebert MP, Szokodi D, Wehvnignh B, Malfertheiner P, Ehninger G, Bayerdoerffer E. Expression of cyclin genes in human gastric cancer and in first degree relatives. Chin Med J (Engl). 2002;115:710-715. [PubMed] |
| 5. | Hamajima N, Goto Y, Nishio K, Tanaka D, Kawai S, Sakakibara H, Kondo T. Helicobacter pylori eradication as a preventive tool against gastric cancer. Asian Pac J Cancer Prev. 2004;5:246-252. [PubMed] |
| 6. | Selbach M, Moese S, Backert S, Jungblut PR, Meyer TF. The Helicobacter pylori CagA protein induces tyrosine dephosphorylation of ezrin. Proteomics. 2004;4:2961-2968. [PubMed] [DOI] |
| 7. | Maurice-Duelli A, Ndoye A, Bouali S, Leroux A, Merlin JL. Enhanced cell growth inhibition following PTEN nonviral gene transfer using polyethylenimine and photochemical internalization in endometrial cancer cells. Technol Cancer Res Treat. 2004;3:459-465. [PubMed] [DOI] |
| 8. | Ohigashi T, Mizuno R, Nakashima J, Marumo K, Murai M. Inhibition of Wnt signaling downregulates Akt activity and induces chemosensitivity in PTEN-mutated prostate cancer cells. Prostate. 2004;62:61-68. [PubMed] [DOI] |
| 10. | Khan S, Kumagai T, Vora J, Bose N, Sehgal I, Koeffler PH, Bose S. PTEN promoter is methylated in a proportion of invasive breast cancers. Int J Cancer. 2004;112:407-410. [PubMed] [DOI] |
| 11. | Zhou YJ, Xiong YX, Wu XT, Shi D, Fan W, Zhou T, Li YC, Huang X. Inactivation of PTEN is associated with increased angiogenesis and VEGF overexpression in gastric cancer. World J Gastroenterol. 2004;10:3225-3229. [PubMed] [DOI] |
| 12. | Yang L, Kuang LG, Zheng HC, Li JY, Wu DY, Zhang SM, Xin Y. PTEN encoding product: a marker for tumorigenesis and progression of gastric carcinoma. World J Gastroenterol. 2003;9:35-39. [DOI] |
| 13. | Zheng HC, Li YL, Sun JM, Yang XF, Li XH, Jiang WG, Zhang YC, Xin Y. Growth, invasion, metastasis, differentiation, angiogenesis and apoptosis of gastric cancer regulated by expression of PTEN encoding products. World J Gastroenterol. 2003;9:1662-1666. [PubMed] [DOI] |
| 14. | Sun H, Zheng H, Yang X, Wu D, Zhang S, Kuang L, Xin Y. Expression of PTEN and Caspase-3 and their clinicopathological significance in primary gastric malignant lymphoma. Chin Med Sci J. 2004;19:19-24. [PubMed] |
| 15. | Tsugawa K, Jones MK, Akahoshi T, Moon WS, Maehara Y, Hashizume M, Sarfeh IJ, Tarnawski AS. Abnormal PTEN expression in portal hypertensive gastric mucosa: a key to impaired PI 3-kinase/Akt activation and delayed injury healing? FASEB J. 2003;17:2316-2318. [PubMed] [DOI] |
| 16. | Shcherbata HR, Althauser C, Findley SD, Ruohola-Baker H. The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions. Development. 2004;131:3169-3181. [PubMed] [DOI] |
| 18. | Cam WR, Masaki T, Shiratori TY, Kato N, Okamoto M, Yamaji Y, Igarashi K, Sano T, Omata M. Activation of cyclin E-dependent kinase activity in colorectal cancer. Dig Dis Sci. 2001;46:2187-2198. [PubMed] [DOI] |
| 19. | Bardon S, Foussard V, Fournel S, Loubat A. Monoterpenes inhibit proliferation of human colon cancer cells by modulating cell cycle-related protein expression. Cancer Lett. 2002;181:187-194. [DOI] |
| 20. | Miehlke S, Yu J, Ebert M, Szokodi D, Vieth M, Kuhlisch E, Buchcik R, Schimmin W, Wehrmann U, Malfertheiner P. Expression of G1 phase cyclins in human gastric cancer and gastric mucosa of first-degree relatives. Dig Dis Sci. 2002;47:1248-1256. [PubMed] [DOI] |
| 23. | Lin HJ, Xue J, Bai Y, Wang JD, Zhang YL, Zhou DY. Pathogenicty and immune prophylaxis of cag pathogenicity island gene knockout homogenic mutants. World J Gastroenterol. 2004;10:3289-3291. [PubMed] [DOI] |
| 24. | Sugiyama T, Asaka M. Helicobacter pylori infection and gastric cancer. Med Electron Microsc. 2004;37:149-157. [PubMed] [DOI] |
| 25. | Torres MM, Acosta CP, Sicard DM, Groot de Restrepo H. [Genetic susceptibility and risk of gastric cancer in a human population of Cauca, Colombia]. Biomedica. 2004;24:153-162. [PubMed] [DOI] |
| 27. | Konturek PC, Kania J, Kukharsky V, Raithel M, Ocker M, Rembiasz K, Hahn EG, Konturek SJ. Implication of peroxisome proliferator-activated receptor gamma and proinflammatory cytokines in gastric carcinogenesis: link to Helicobacter pylori-infection. J Pharmacol Sci. 2004;96:134-143. [PubMed] [DOI] |
| 28. | Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 Overexpression in Human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src dependent NF-{kappa}B activation. Mol Pharmacol. 2004; [Epub ahead of print]. [DOI] |
| 30. | Nagata J, Kijima H, Takagi A, Ito M, Goto K, Yamazaki H, Nakamura M, Mine T, Ueyama Y. Helicobacter pylori induces chronic active gastritis in p53-knockout mice. Int J Mol Med. 2004;13:773-777. [DOI] |