修回日期: 2004-07-20
接受日期: 2004-07-27
在线出版日期: 2004-11-15
目的: 探讨胃癌细胞bcl-2基因表达水平与肿瘤细胞增生活性及细胞凋亡程度的关系.
方法: 胃癌组织53例, 用原位杂交及免疫组化染色法分别检测bcl-2 mRNA, bcl-2蛋白和增生细胞核抗原(PCNA)的表达, 并采用凋亡细胞原位检测方法对组织切片中的凋亡细胞进行观察和比较.
结果: 胃癌组织表达bcl-2 mRNA 41例(77.4%), 表达Bcl-2蛋白43例(81.1%), χ2检验表明两种方法检测阳性率差异无显著性. 胃癌53例增生期细胞标记物PCNA表达及凋亡细胞的阳性率均为100%, 细胞凋亡和细胞增生指数呈显著性负相关(r = -0.993, P<0.01). 随胃癌细胞Bcl-2蛋白表达水平升高, PCNA阳性细胞指数相应增加, 肿瘤凋亡细胞指数则相应减少, Bcl-2蛋白+++组与++组间(t = 2.552, 2.699, P<0.05)及前两组分别与-组和+组间(t = 4.487, 3.975, 2.807, 3.094, 4.885, 5.816, 3.404, 3.895, P<0.01)凋亡和增生细胞指数差异均有显著性.
结论: 胃癌细胞bcl-2基因高表达可引起细胞凋亡减少与过度增生.
引文著录: 刘海峰, 刘为纹, 王国安, 滕小春, 陈刚, 汪兴伟, 何俊堂, 姜利国. 胃癌组织bcl-2基因表达与细胞凋亡和增生的关系. 世界华人消化杂志 2004; 12(11): 2543-2546
Revised: July 20, 2004
Accepted: July 27, 2004
Published online: November 15, 2004
AIM: To investigate the relationship between bcl -2 gene expression levels in human gastric carcinoma and the frequency of tumor cell proliferation activity and apoptosis.
METHODS: In situ hybridization and immunohistochemistry methods were used to study the frequencies of expressions of bcl -2 gene and nuclear antigen of proliferating cells (PCNA) in 53 gastric carcinomas. Meanwhile, an in situ apoptotic cell detection (TUNEL method) was adopted to compare the number of apoptotic cells and PCNA with the Bcl -2 protein expression in each case.
RESULTS: Of the 53 gastric carcinomas, 41 and 43 expressed bcl -2 mRNA and Bcl -2 protein, which were count for 77.4% and 81.1% respectively. There was no significant difference between the positive rates obtained by these two methods. The apoptotic index of gastric carcinomas negatively related to PCNA index (r = -0.993, P < 0.01). With the increase of Bcl-2 protein expression , the cell proliferating activity increased but the apoptosis decreased in the tumor cells. Significant difference of cell proliferation and apoptosis existed between +++ group and ++ group of Bcl -2 protein expression (t = 2.552, 2.699, P < 0.05) as well as between the former two groups and the -, + group (t = 4.487, 3.975, 2.807, 3.094, 4.885, 5.816, 3.404, 3.895, P < 0.01) respectively.
CONCLUSION: The overexpression of bcl -2 gene inhibits cell apoptosis and excessive cell proliferation in the development and progress of gastric carcinomas.
- Citation: Liu HF, Liu WW, Wang GA, Teng XC, Chen G, Wang XW, He JT, Jiang LG. Relationship between bcl-2 gene expression and cell proliferation and apoptosis in human gastric carcinomas. Shijie Huaren Xiaohua Zazhi 2004; 12(11): 2543-2546
- URL: https://www.wjgnet.com/1009-3079/full/v12/i11/2543.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i11.2543
胃癌的发生发展不仅与肿瘤细胞的增生而且与其凋亡有密切关系[1-10]. bcl-2是细胞凋亡的重要调控基因, 已知bcl-2基因的异位和/或过度表达所致的细胞凋亡异常减少与多种肿瘤的发生发展密切相关[11-14]. 迄今, bcl-2基因在胃癌前病变及胃癌中的表达状况及其与细胞凋亡和增生的关系已有少数文献报道. 为进一步探讨胃癌细胞bcl-2基因表达水平与肿瘤细胞增生活性及细胞凋亡程度的关系, 我们采用mRNA原位杂交和免疫组织化学染色技术从核酸转录、蛋白表达两个水平对胃癌组织中的bcl-2基因表达状况进行检测, 并应用细胞凋亡原位检测方法和免疫组化染色对胃癌组织中细胞凋亡、增生的情况进行观察, 探讨其与Bcl-2蛋白表达之间可能的关系.
我院1994/1996年胃远端癌手术切除标本53例, 术前均未接受化放疗或免疫治疗. 每份标本的一半立即用冷冻包埋剂(OCT)包埋, 液氮快速冷冻, -70 ℃冰箱保存. 另一半组织标本经40 g/L甲醛固定, 石蜡包埋, 4 μm厚连续切片, 并经HE染色病理诊断证实. 原位杂交前, 将冷藏标本取出复温至-23 ℃, 用恒冷切片机做6 μm连续冷冻切片, 室温干燥10 min, -70 ℃保存备用. PCNA及bcl-2单克隆抗体均购自Santa Cruz公司; S-P免疫染色试剂盒购于福建迈新生物工程公司; 地高辛标记及检测试剂盒以及细胞凋亡原位检测试剂盒均为Boehringer Mannheim公司产品. bcl-2寡核苷酸探针由中科院上海生物工程研究中心合成. 探针序列为: 5'-GGGAAACACCAGAATCAAGT-3'. 探针标记按试剂盒说明进行, 探针标记后分别作探针显色灵敏度及杂交灵敏度检测.
1.2.1 原位杂交: 所用载玻片和盖玻片经认真清洗, 高压消毒, 载玻片上涂多聚赖氨酸(1 g/L), 室温干燥, 石蜡组织直接贴于载玻片上, 65 ℃烤片3 h, 二甲苯脱蜡, 乙醇逐级入水后, 用100 mg/L蛋白酶K消化, 37 ℃ 20 min; 4 mL/L多聚甲醛后固定5 min, 依次用2×SSC、三蒸水处理后, 乙醇逐级脱水; 滴加预杂交液(500 mL/L甲酰胺; 6×SSC, 5 mL/L SDS, 5×Danhardt溶液: 聚乙烯吡咯烷酮1 g/L, 牛血清白蛋白1 g/L, 水溶性聚蔗糖-400 1 g/L, 200 mg/L ssDNA), 42 ℃预杂交2 h, 吸弃载玻片上预杂交液, 每片滴加50 μL杂交液, 置于湿盒中42 ℃杂交过夜. 杂交后按下述程序严格冲洗: 0.1 mL/L SDS 2×SSC 42 ℃ 10 min 2次, 500 mL/L甲酰胺2×SSC室温10 min 2次, 500 mL/L甲酰胺0.5×SSC室温10 min 2次, 0.2×SSC室温5 min 1次, 0.1×SSC室温2 min 1次. 然后按试剂盒说明进行显色, 结果满意后水洗终止, 封片. 除阳性对照外, 设无探针阴性对照及RNase处理后的阴性对照.
1.2.2 免疫组化染色和TUNEL染色: 石蜡切片常规脱蜡、水化, 继以免疫组化试剂盒检测, 染色程序按SP法操作常规进行, 抗PCNA及bcl-2单抗1:100稀释. 以PBS替代一抗作为空白对照, 正常血清替代一抗作为替代对照. 用已知阳性切片作为阳性对照. 石蜡切片脱蜡, 逐级入水, 蛋白酶K(20 mg/L)37 ℃消化20 min, PBS(pH7.4)洗5 min, 3次; 滴加TUNEL反应液37 ℃孵育1 h, PBS洗5 min, 3次; 滴加抗荧光素抗体-过氧化物酶联结物37 ℃ 30 min, PBS洗5 min, 3次; DAB显色, 苏木素复染, 封片. 阴性对照设以PBS替代TUNEL反应液的空白对照, 阳性对照切片经DNaseI预处理10 min, 再接上述染色步骤. 光镜下观察TUNEL染色及PCNA免疫组化染色切片的显色反应, 数5个以上高倍视野, 不少于500个细胞, 计数阳性细胞数, 分别作为TUNEL阳性细胞(凋亡细胞)指数及PCNA阳性细胞(增生细胞)指数. 用上述同样方法计数bcl-2 mRNA和Bcl-2蛋白阳性肿瘤细胞数, 将其分为4个等级: -, 无阳性细胞; +, 阳性细胞<25%; ++, 阳性细胞占25-50%; +++, 阳性细胞>50% .
统计学处理 采用χ2检验、t检验和相关分析进行统计学处理.
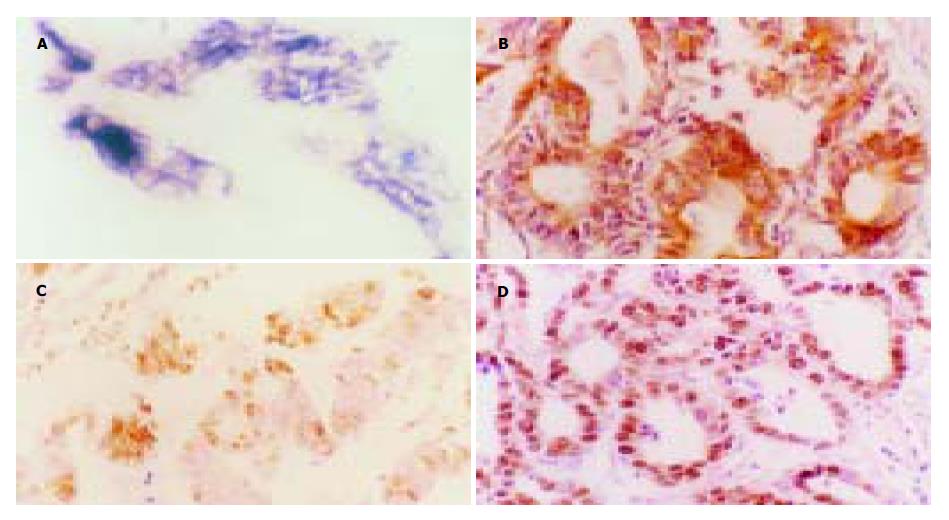
bcl-2 mRNA原位杂交阳性信号为紫蓝色或棕蓝色颗粒, 主要集中在胞质内(图1A). Bcl-2 蛋白免疫组化阳性信号呈棕黄色颗粒, 位于细胞质内和/或细胞膜上, 未见核内表达(图1B). 在53例胃癌中, 有41例(77.4%)不同程度地表达bcl-2 mRNA, Bcl-2 mRNA -、+、++、+++者分别占22.6%(12/53), 11.3%(6/53), 37.7%(20/53)和28.3%(15/53). 不同程度地表达Bcl-2蛋白43例(81.1%), Bcl-2蛋白 -, +, ++, +++者分别占18.9%(10/53), 11.3%(6/53), 39.6%(21/53)和30.2%(16/53). 经四格表χ2检验表明, 原位杂交及免疫组化两种方法对Bcl-2基因表达检测结果一致性非常显著(P<0.01), 53例标本中两种方法检测结果一致者占86.8%(46/53), 同时发现两种方法的差异则未达到显著界限(P>0.05).
TUNEL染色阳性反应物质呈棕黄色, 位于细胞核内, 浓缩的核质紧贴于核膜, 或核质呈现均匀的染色, 凋亡细胞呈弥漫性、簇状和/或散在分布(图1C); 未凋亡细胞非特异性染色极浅, 而坏死组织染色呈片状, 细胞轮廓不清, 染色呈均一深染的棕黄色斑片. PCNA染色阳性物质呈棕黄色颗粒状均匀分布, 主要位于细胞核内, PCNA阳性细胞在胃癌组织中呈弥漫性分布(图1D). 53例胃癌细胞凋亡和PCNA表达的阳性率均为100%, 细胞凋亡指数为5.8% , PCNA指数为47.5%, 直线相关分析证实二者间呈显著性负相关(r =-0.993, P<0.01). PCNA阳性指数随肿瘤细胞Bcl-2蛋白表达水平升高而相应增加, 肿瘤细胞凋亡指数随肿瘤细胞Bcl-2蛋白表达水平升高而相应减少, 除Bcl-2蛋白-组与+组间无统计学差异外, 其余各组间PCNA和肿瘤细胞凋亡指数的差异均有显著性, 见表1. 经连续切片观察, Bcl-2蛋白表达与细胞凋亡和PCNA阳性细胞在多数病例具有同域性.
bcl-2基因是Tsujimoto et al在1985年研究人滤泡性非霍奇金淋巴瘤患者染色体t(14; 18)易位时发现的一个基因, 编码两个结构相近的蛋白质片段: Bcl-2 β(26 000)和 Bcl-2 β(21 000). 正常情况下Bcl-2基因定位于人染色体18q21, 其编码产物Bcl-2蛋白可通过拮抗野生型p53蛋白的促凋亡作用, 将c-myc蛋白由促凋亡逆转为抑制凋亡及阻断caspase酶类活化的上游信号传递通路等作用抑制多种因素诱发的细胞凋亡, 参与细胞增生和凋亡动态平衡的调控[15-17]. bcl-2基因表达异常增加可使已有基因异常改变的细胞逃避凋亡, 由此引起的基因异常事件的累积是导致细胞转化和肿瘤形成的必要前提. Bcl-2蛋白对细胞凋亡具有直接调节的作用, 能够抑制许多因素引起的细胞凋亡, 但并不能刺激增生, 而是促进非增生细胞的存活[18-19]. 研究发现, Bcl-2不仅存在于T及B淋巴细胞, 在未成熟的造血细胞、上皮细胞、神经细胞及多种癌细胞中均有表达[20-22]. 我们发现, 53例胃癌组织中bcl-2 mRNA和蛋白的阳性率分别为77.4%和81.1%, bcl-2蛋白与bcl-2 mRNA检测结果呈显著性正相关, 提示胃癌细胞普遍存在Bcl-2蛋白翻译及核酸转录水平的过度表达, bcl-2基因异常表达可能与胃癌的发生、发展及浸润性生长能力的获得密切相关.
我们既往的研究工作已经证实, 胃黏膜癌变过程中不仅存在活跃的细胞增生, 而且存在细胞凋亡的异常, 细胞过度增生和(或)细胞凋亡减少是胃癌发生的生物学基础[23-30]. 但胃黏膜癌变过程中细胞凋亡和增生异常的机制尚不明确, 可能与细胞凋亡相关基因调控失常有关. 本组53例胃癌增生期细胞标记物PCNA表达及凋亡细胞的阳性率均为100%, 细胞凋亡和细胞增生指数呈显著性负相关. 随胃癌细胞Bcl-2蛋白表达水平升高, PCNA阳性指数相应增加, 肿瘤细胞凋亡指数则相应减少, 除Bcl-2蛋白-组与+组间外, 其余各组间PCNA和肿瘤细胞凋亡指数的差异均有显著性. 研究结果提示, bcl-2 基因表达异常增加可抑制胃癌细胞凋亡, 抑制强度随胃癌细胞bcl-2基因表达水平升高而相应增加. 虽然bcl-2基因表达异常增加可能不是胃癌细胞过度增生的直接原因, 但二者之间的协同作用可引起细胞无限蓄积, 并在胃癌发生及发展过程中起重要作用.
编辑: N/A
| 1. | Jang TJ, Kim JR. Proliferation and apoptosis in gastric antral epithelial cells of patients infected with Helicobacter pylori. J Gastroenterol. 2000;35:265-271. [PubMed] [DOI] |
| 2. | Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779-782. [PubMed] [DOI] |
| 3. | Chan AO, Wong BC, Lam SK. Gastric cancer: past, present and future. Can J Gastroenterol. 2001;15:469-474. [PubMed] [DOI] |
| 4. | Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28-37. [PubMed] [DOI] |
| 5. | Wang J, Chi DS, Kalin GB, Sosinski C, Miller LE, Burja I, Thomas E. Helicobacter pylori infection and oncogene expressions in gastric carcinoma and its precursor lesions. Dig Dis Sci. 2002;47:107-113. [PubMed] [DOI] |
| 6. | Ebert MP, Leodolter A, Malfertheiner P. Novel strategies in the prevention of gastric cancer. Hepatogastroenterology. 2001;48:1569-1571. [PubMed] |
| 7. | El-Omar EM, Chow WH, Rabkin CS. Gastric cancer and H. pylori: Host genetics open the way. Gastroenterology. 2001;121:1002-1004. [PubMed] [DOI] |
| 8. | Maeda S, Yoshida H, Mitsuno Y, Hirata Y, Ogura K, Shiratori Y, Omata M. Analysis of apoptotic and antiapoptotic signalling pathways induced by Helicobacter pylori. Gut. 2002;50:771-778. [PubMed] [DOI] |
| 9. | Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796-800. [PubMed] [DOI] |
| 10. | Hamajima N, Matuo K, Watanabe Y, Suzuki T, Nakamura T, Matsuura A, Yamao K, Ohashi K, Tominaga S. A pilot study to evaluate stomach cancer risk reduction by Helicobacter pylori eradication. Am J Gastroenterol. 2002;97:764-765. [PubMed] [DOI] |
| 11. | Yan J, Xu YH. Tributyrin inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2003;9:660-664. [PubMed] [DOI] |
| 12. | Zhou HB, Zhu JR. Paclitaxel induces apoptosis in human gastric carcinoma cells. World J Gastroenterol. 2003;9:442-445. [PubMed] [DOI] |
| 13. | Chen YX, Zhong XY, Qin YF, Bing W, He LZ. 15d-PGJ2 inhibits cell growth and induces apoptosis of MCG-803 human gastric cancer cell line. World J Gastroenterol. 2003;9:2149-2153. [PubMed] [DOI] |
| 14. | Xu AH, Chen HS, Sun BC, Xiang XR, Chu YF, Zhai F, Jia LC. Therapeutic mechanism of ginkgo biloba exocarp polysaccharides on gastric cancer. World J Gastroenterol. 2003;9:2424-2427. [PubMed] |
| 15. | Lan J, Xiong YY, Lin YX, Wang BC, Gong LL, Xu HS, Guo GS. Helicobacter pylori infection generated gastric cancer through p53-Rb tumor-suppressor system mutation and telomerase reactivation. World J Gastroenterol. 2003;9:54-58. [PubMed] [DOI] |
| 16. | Liu JR, Chen BQ, Yang YM, Wang XL, Xue YB, Zheng YM, Liu RH. Effect of apoptosis on gastric adenocarcinoma cell line SGC-7901 induced by cis-9, trans-11-conjugated linoleic acid. World J Gastroenterol. 2002;8:999-1004. [PubMed] [DOI] |
| 17. | Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792-796. [PubMed] [DOI] |
| 18. | Liu S, Wu Q, Ye XF, Cai JH, Huang ZW, Su WJ. Induction of apoptosis by TPA and VP-16 is through translocation of TR3. World J Gastroenterol. 2002;8:446-450. [PubMed] [DOI] |
| 19. | Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NF-kB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J Gastroenterol. 2002;8:431-435. [PubMed] [DOI] |
| 20. | Heckman CA, Mehew JW, Boxer LM. NF-kappaB activates Bcl-2 expression in t(14;18) lymphoma cells. Oncogene. 2002;21:3898-3908. [PubMed] [DOI] |
| 21. | Gobé G, Rubin M, Williams G, Sawczuk I, Buttyan R. Apoptosis and expression of Bcl-2, Bcl-XL, and Bax in renal cell carcinomas. Cancer Invest. 2002;20:324-332. [PubMed] [DOI] |
| 22. | Satomi D, Takiguchi N, Koda K, Oda K, Suzuki H, Yasutomi J, Ishikura H, Miyazaki M. Apoptosis and apoptosis-associated gene products related to the response to neoadjuvant chemotherapy for gastric cancer. Int J Oncol. 2002;20:1167-1171. [PubMed] [DOI] |
| 23. | Sun WH, Yu Q, Shen H, Ou XL, Cao DZ, Yu T, Qian C, Zhu F, Sun YL, Fu XL. Roles of Helicobacter pylori infection and cyclooxygenase-2 expression in gastric carcinogenesis. World J Gastroenterol. 2004;10:2809-2813. [PubMed] [DOI] |
| 24. | De Luca A, Iaquinto G. Helicobacter pylori and gastric diseases: a dangerous association. Cancer Lett. 2004;213:1-10. [PubMed] [DOI] |
| 25. | Yang GF, Deng CS, Xiong YY, Gong LL, Wang BC, Luo J. Expression of nuclear factor-kappa B and target genes in gastric precancerous lesions and adenocarcinoma: association with Helicobactor pylori cagA (+) infection. World J Gastroenterol. 2004;10:491-496. [PubMed] [DOI] |
| 26. | Liu HF, Liu WW, Fang DC, Yang SM, Wang RQ. Bax geng expression and its relationship with apoptosis in human gastric carcinoma and paracancerous tissues. Shijie Huaren Xiaohua Zazhi. 2000;8:665-668. |
| 27. | Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15-17. [PubMed] [DOI] |