修回日期: 2004-08-09
接受日期: 2004-08-25
在线出版日期: 2004-10-15
目的: 研究乙醛对肝星状细胞(HSC)细胞外基质(ECM)及其降解酶表达的影响及中药抗纤复方Ⅰ号(KXI)的干预作用.
方法: 大鼠HSC体外原代及传代培养, 大鼠灌以KXI制备药物血清, 用乙醛及药物血清处理HSC. 以放射免疫分析法及RT-PCR分别检测培养上清中层粘连蛋白(LN)及HSC中a1(I), a1(IV)型胶原及基质金属蛋白酶-1, 2(MMP-1, 2) mRNA的表达.
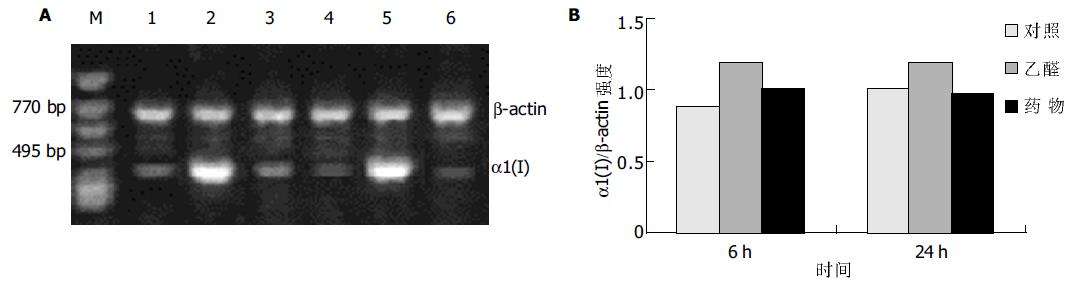
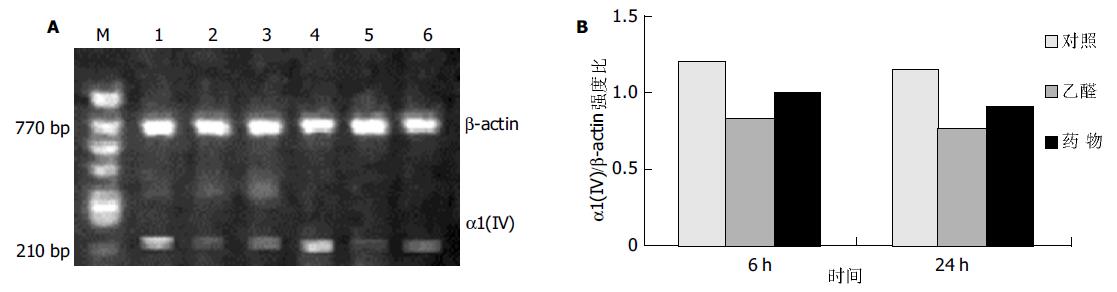
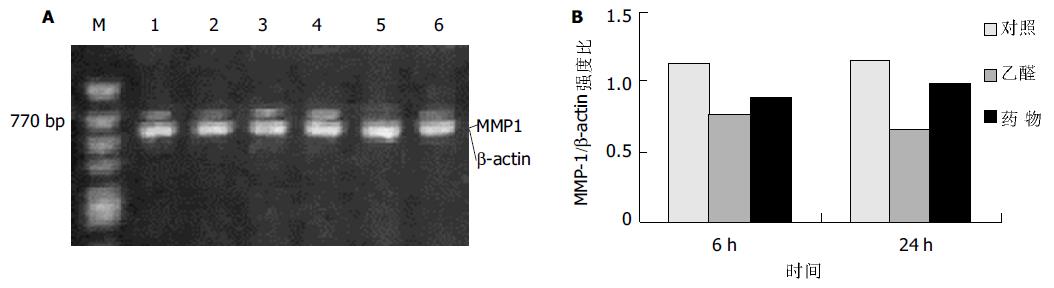
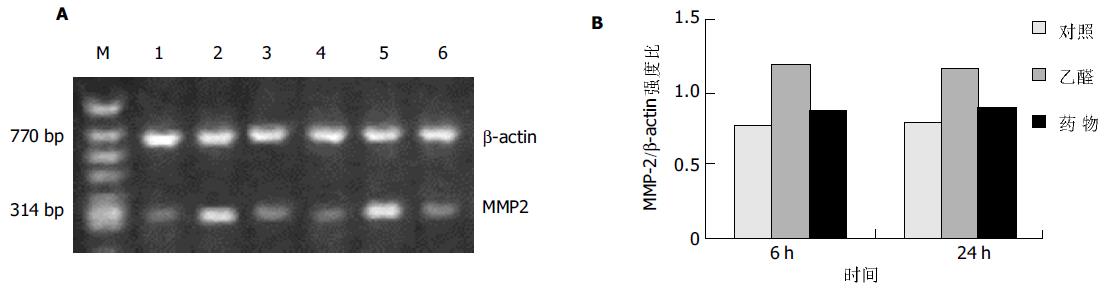
结果: 培养上清中LN含量在100 mmol/L乙醛处理24 h后显著增加 (52.0±12.1 vs 10.0±0.3, P <0.01), 100 mL/L药物血清起抑制作用(19.2±7.8 vs 52.0±12.1, P <0.01); 100 mmol/L乙醛刺激HSCa1 (I)型胶原及MMP-2 mRNA的表达明显增强, 100 mL/L药物血清抑制其作用; 100 mmol/L乙醛减弱HSCa1(IV)型胶原及MMP-1 mRNA的表达, 100 mL/L药物血清则使二者表达有所增强.
结论: KXI能抑制乙醛刺激的HSC中LN的分泌, a1(I)型胶原及MMP-2 mRNA的表达; 而增强乙醛所抑制的HSC中a1(IV)型胶原及MMP-1 mRNA的表达.
引文著录: 林红, 张义侠, 李异玲, 崔巍, 王炳元, 傅宝玉. 抗纤复方Ⅰ号对肝星状细胞细胞外基质及其降解酶表达的影响. 世界华人消化杂志 2004; 12(10): 2472-2475
Revised: August 9, 2004
Accepted: August 25, 2004
Published online: October 15, 2004
N/A
- Citation: N/A. N/A. Shijie Huaren Xiaohua Zazhi 2004; 12(10): 2472-2475
- URL: https://www.wjgnet.com/1009-3079/full/v12/i10/2472.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i10.2472
在酒精性肝纤维化和肝硬化形成过程中, 肝星状细胞(HSC)起重要作用, 中药复方的多途径、多层次、多靶点的综合药理作用在抗肝纤维化治疗中显示出独特的优势. 我们观察了抗纤复方Ⅰ号(KXI)对乙醛刺激的大鼠HSC分泌LN, a1(I), a1(IV)型胶原及合成MMP-1, 2的影响, 以期阐述KXI抗酒精性肝纤维化的部分作用机制, 为临床酒精性肝病 (ALD) 的中药治疗提供更多的理论依据.
中药KXI(由丹参、黄芪、红花、汉防己、葛根、桃仁、甘草等10味药物组成)购于省医药公司; 大鼠肝脏原位灌流消化所需的链霉蛋白酶、IV型胶原酶及密度梯度递质Nycodenz均为Sigma产品; Desmin抗体、免疫细胞化学试剂盒购自华美公司; LN放射免疫测定试剂盒购于上海海军医学研究所; RT-PCR试剂盒购于大连宝生物工程有限公司; a1(I)、a1(IV)型胶原、MMP-1、2及β-actin引物均由北京奥科公司合成. 用2.7 kg/L KXI生药11 mL/kg给大鼠灌服, 每天1次, 持续给药1 wk, 末次给药后1 h无菌条件下由下腔静脉取血, 取血清, 56 ℃灭活30 min, 分装-20 ℃冻存. 另选正常大鼠喂以等量生理盐水, 以同样方法制备正常血清作对照用. 肝星状细胞分离、培养及鉴定参照宋少刚et al[1]报道的大鼠肝脏原位灌流消化的方法分离HSC. 细胞得率约为1×107/肝, 经台盼蓝染色细胞存活率90%以上. 将细胞爬片用兔抗人Desmin抗体、SP法作免疫细胞化学染色以鉴定HSC, 胞质阳染者为HSC, 传代的HSC纯度>98%. 本次实验采用传2-4代HSC.
放免法测定LN含量以1×106/瓶浓度接种在培养瓶(25 cm2)中的传代的HSC长满单层后, 分为正常血清对照组、正常血清+乙醛组及药物血清+乙醛组. 正常血清及药物血清加入无血清DMEM培养液中, 制成浓度为100 mL/L的正常血清及药物血清孵育液, 乙醛组加入乙醛后使终浓度为100 mmol/L, 培养瓶均用封口膜封严, 培养24 h后收集培养上清, 严格按说明操作. JC-1200放射免疫g计数仪上直接测定LN含量(mg/L), 取均值. HSC处理后, 提取6 h和24 h的总RNA并逆转录成cDNA. 引物序列及扩增产物片段(见表1). 总体积25 mL, a1(I)、a1(IV)型胶原反应条件: 94 ℃ 3 min变性→94 ℃40 s→a1(I)62 ℃ 1 min (a1(IV) 65 ℃ 1 min)→72 ℃1 min, 共35个循环, 72 ℃ 7 min终止反应; MMP-1反应条件: 94 ℃ 3 min变性→94 ℃ 30 s→55 ℃ 1 min→72 ℃ 2 min, 共35个循环, 72 ℃ 5 min终止反应; MMP-2反应条件: 94 ℃ 3 min变性→94 ℃ 45 s→52 ℃ 1 min→72 ℃ 1 min, 共35个循环, 72 ℃ 7 min终止反应. PCR产物经20 g/L琼脂糖凝胶电泳、摄片、密度扫描(1D Kodak成像分析系统), 然后用其表达量与对应的β-actin表达量进行比较.
| 引物名称 | 引物序列 | PCR产物位置 |
| MMP-1-F | 5'-TGGAGCCCTGATGTTTCCCATCTA -3' | 753 bp |
| MMP-1-R | 5'-CGTAACCCTAATAGTCTTTGTCCATA -3' | |
| MMP-2-F | 5'-TCAACGGTCGGGAATACA -3' | 307 bp |
| MMP-2-R | 5'-CCCACAGTGGACATAGCG-3' | |
| a1 ( I )-F | 5'-CCGTGGTGACAAGGGTGAGACAG -3' | 471 bp |
| a1 ( I )-R | 5'-TCAGGGCTGCGGATGTTCTCA -3' | |
| a1 (IV) -F | 5'-CAGGTCTCTGCTCAGAGCCACCG -3' | 222 bp |
| a1 (IV)-R | 5'-CGGCTGCGCTCCTCGTGG -3' | |
| β-actin-F | 5'-GATTGCCTCAGGACATTTCTG-3' | 690 bp |
| β-actin-R | 5'-GATTGCTCAGGACATTTCTG-3' |
统计学处理 各组数据以均值±标准差(mean±SD)表示, 两两均数的比较用t检验.
100 mmol/L乙醛及100 mL/L药物血清对HSC形态和生长无影响. 正常血清对照组LN分泌量为(10.0±0.3 mg/L), 正常血清+乙醛组为(52.0±12.1 mg/L)(与对照组比 P <0.01, t = 4.53), 药物血清+乙醛组为(19.2±7.8 mg/L)(与正常血清+乙醛组比 P <0.01, t = 4.61).
RT-PCR结果显示, 乙醛浓度为100 mmol/L时, 刺激HSC 6 ha1(I)型胶原mRNA表达显著增加, 至24 h仍有较强的信号, 100 mL/L药物血清能明显抑制a1(I)型胶原mRNA表达(图1); 而乙醛刺激HSC 6 h后, a1(IV)型胶原mRNA表达明显减弱, 100 mL/L药物血清则使其表达有所增强(图2).
MMP-1在对照组HSC中有弱表达, 乙醛处理6 h后表达明显减弱, 而100 mL/L药物血清使其表达信号有所增强(图3); HSC中MMP-2 mRNA信号在乙醛刺激6 h 后显著增高, 持续至24 h, 药物血清能明显抑制其表达的增高(图4).
ECM合成增加、降解减少导致肝纤维化的发生和发展[2-3]. ECM包括胶原、非胶原糖蛋白及蛋白多糖等, 胶原是ECM的主要成分. HSC的活化是肝纤维化发生的中心环节, 是分泌合成胶原的主要细胞[4]. LN为非胶原蛋白, 与IV型胶原相连构成非连续性的功能性基底膜. 对维持肝脏正常结构、微循环及肝脏细胞功能有重要作用, 亦使HSC处于静止状态. DMN诱导的大鼠肝纤维化[5], LN和I型胶原明显增加; ALD者I型胶原表达增加[6-7], PIIINP, HA及LN表达亦增加[8-12], 戒酒后LN明显下降[13]. 尽管也有相反的报道[14-15]. MMPs是体内生理或病理条件下降解ECM的主要酶系, 其在肝纤维化发生和发展中起重要的作用[16-17]. MMPs可分成间质胶原酶(MMP-1)、明胶酶又称IV型胶原酶(包括MMP-2和MMP-9)及基质分解素三大类, MMP-1主要降解I、III型胶原, IV型胶原酶主要降解IV型胶原.目前已发现有三种MMPs可以降解正常内皮下基质: MMP-2, MMP-9和MMP-3, 若他们活性增高, 同时MMP-1活性下降, 可引起胶原纤维沉积于Disse腔, 导致肝窦"毛细血管化", 血液交换发生障碍, 肝细胞功能受损, 并活化HSC, 产生大量ECM, 引起肝纤维化.研究表明, 肝硬化患者MMP-2, 9及胶原表达明显增加[18-20], 有人提出PIIINP及MMP-1, 2可作为评价肝硬化轻重的指标[21]. ALD者肝窦周出现纤维化, 肝窦毛细血管化[22], 肝脏MMP-2, 9表达增加[23].
在ALD发病机制中, 乙醇在肝细胞内代谢产生的毒性代谢产物乙醛、乙醛蛋白加合物及引起的代谢紊乱是导致酒精性肝损伤的主要原因[24]. 应用ALD动物模型[25]研究发现, 随着ALD的进展, MMP-2及MMP-9水平呈进行性增加[26], TIMP-1表达亦随病变进展而逐渐增加[27]; 乙醛能刺激HSC增生, PCIII分泌明显增加[28]; 随着给大鼠乙醇灌胃时间的延长, 肝窦内皮细胞窗孔数减少, 直径减小, 进而消失, 成为肝窦毛细血管化的基础[29]. 本结果表明, MMP-1在对照组HSC中表达较弱, 而MMP-2则表达相对较强, 100 mmol/L乙醛能上调MMP-2 mRNA表达, 抑制MMP-1mRNA表达; 与之相反, 经乙醛刺激后a1(IV)型胶原mRNA表达减弱, a1(I)型胶原mRNA表达增强, 这与国外研究结果基本一致[30]; 同时乙醛能增加LN的分泌. 由此提示乙醛通过增加HSC中MMP-2表达, 减少MMP-1表达, 促进ECM中I型胶原积聚、LN的分泌及Disse腔中IV型胶原的降解, 破坏正常基底膜, 加速肝窦"毛细血管化"及肝纤维化的发生. 而活血化瘀类中药在酒精性肝病动物模型及人肝硬化防治中表现出一定作用, Zhu et al[31]报道861合剂能抑制实验鼠肝纤维化中MMP-2mRNA表达, 但其活性增高; 另有研究表明[32], 中药能通过减少HSC中TIMP表达而提高MMP-2及IV型胶原的表达. 我们的研究显示[33-34], 由活血化瘀类药物组成的KXI有良好的抗大鼠ALD的作用, 他能抑制血清中HA的增高, 减少肝组织中丙二醛的产生, 并保持超氧化物歧化酶高水平不降, 抑制HSC结构的破坏及肝脏纤维条索的形成. 我们发现KXI可能通过上调MMP-1表达, 下调MMP-2表达, 从而减少I型胶原积聚、LN的分泌及IV型胶原的降解, 从而减缓或抑制ECM的积聚、肝窦"毛细血管化"及ALD的发生和发展.
| 1. | 宋 少刚, 杨 雁, 陈 敏珠. 大鼠肝贮脂细胞、枯否细胞的同时分离和培养. 中国临床药理学与治疗学. 2000;5:351-353. |
| 2. | Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373-384. [PubMed] [DOI] |
| 3. | Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351-372. [PubMed] [DOI] |
| 4. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [PubMed] [DOI] |
| 5. | Wang XB, Liu P, Tang ZP, Lu X, Liu CH, Hu YY, Xu LM, Gu HT, Liu C. [The role of changes of MMP-2, 9 activity in the development of liver fibrosis in rats]. Zhonghua Gan Zang Bing Za Zhi. 2004;12:267-270. [PubMed] |
| 6. | Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, Cederbaum AI. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem. 2000;275:20136-20145. [PubMed] [DOI] |
| 7. | Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37:336-339. [PubMed] [DOI] |
| 8. | Castera L, Hartmann DJ, Chapel F, Guettier C, Mall F, Lons T, Richardet JP, Grimbert S, Morassi O, Beaugrand M. Serum laminin and type IV collagen are accurate markers of histologically severe alcoholic hepatitis in patients with cirrhosis. J Hepatol. 2000;32:412-418. [PubMed] [DOI] |
| 9. | Kato J, Sato Y, Inui N, Nakano Y, Takimoto R, Takada K, Kobune M, Kuroiwa G, Miyake S, Kohgo Y. Ethanol induces transforming growth factor-alpha expression in hepatocytes, leading to stimulation of collagen synthesis by hepatic stellate cells. Alcohol Clin Exp Res. 2003;27:58S-63S. [PubMed] [DOI] |
| 10. | Nøjgaard C, Johansen JS, Christensen E, Skovgaard LT, Price PA, Becker U; EMALD Group. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol. 2003;39:179-186. [PubMed] [DOI] |
| 11. | Gutiérrez-Ruiz MC, Bucio L, Correa A, Souza V, Hernández E, Gómez-Quiroz LE, Kershenobich D. Metadoxine prevents damage produced by ethanol and acetaldehyde in hepatocyte and hepatic stellate cells in culture. Pharmacol Res. 2001;44:431-436. [PubMed] [DOI] |
| 12. | Stickel F, Urbaschek R, Schuppan D, Poeschl G, Oesterling C, Conradt C, McCuskey RS, Simanowski UA, Seitz HK. Serum collagen type VI and XIV and hyaluronic acid as early indicators for altered connective tissue turnover in alcoholic liver disease. Dig Dis Sci. 2001;46:2025-2032. [PubMed] [DOI] |
| 13. | Ponomarenko Y, Leo MA, Kroll W, Lieber CS. Effects of alcohol consumption on eight circulating markers of liver fibrosis. Alcohol Alcohol. 2002;37:252-255. [PubMed] [DOI] |
| 14. | Cheng ML, Wu J, Zhang WS, Wang HQ, Li CX, Huang NH, Yao YM, Ren LG, Ye L, Li L. Effect of Maotai liquor on the liver: an experimental study. Hepatobiliary Pancreat Dis Int. 2004;3:93-98. [PubMed] |
| 15. | Cheng ML, Wu J, Wang HQ, Xue LM, Tan YZ, Ping L, Li CX, Huang NH, Yao YM, Ren LZ. Effect of Maotai liquor in inducing metallothioneins and on hepatic stellate cells. World J Gastroenterol. 2002;8:520-523. [PubMed] [DOI] |
| 16. | Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245-G249. [PubMed] |
| 17. | Okazaki I, Watanabe T, Hozawa S, Arai M, Maruyama K. Molecular mechanism of the reversibility of hepatic fibrosis: with special reference to the role of matrix metalloproteinases. J Gastroenterol Hepatol. 2000;15 Suppl:D26-D32. [PubMed] [DOI] |
| 18. | Kuyvenhoven JP, Verspaget HW, Gao Q, Ringers J, Smit VT, Lamers CB, van Hoek B. Assessment of serum matrix metalloproteinases MMP-2 and MMP-9 after human liver transplantation: increased serum MMP-9 level in acute rejection. Transplantation. 2004;77:1646-1652. [PubMed] [DOI] |
| 19. | Kuyvenhoven JP, van Hoek B, Blom E, van Duijn W, Hanemaaijer R, Verheijen JH, Lamers CB, Verspaget HW. Assessment of the clinical significance of serum matrix metalloproteinases MMP-2 and MMP-9 in patients with various chronic liver diseases and hepatocellular carcinoma. Thromb Haemost. 2003;89:718-725. [PubMed] |
| 20. | Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113:443-453. [PubMed] |
| 21. | Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, Morel F, Zarski JP. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol. 2004;99:271-279. [PubMed] [DOI] |
| 22. | Xu GF, Wang XY, Ge GL, Li PT, Jia X, Tian DL, Jiang LD, Yang JX. Dynamic changes of capillarization and peri-sinusoid fibrosis in alcoholic liver diseases. World J Gastroenterol. 2004;10:238-243. [PubMed] |
| 23. | Kwon OS, Lim DY, Kwon KA, Chung MG, Park DK, Kim SS, Kim YS, Kwon SY, Koo YS, Kim YK. [Clinical usefulness of plasma activities of gelatinase (matrix metalloproteinase-2 and 9) in chronic liver disease]. Taehan Kan Hakhoe Chi. 2003;9:222-230. [PubMed] |
| 24. | Li CJ, Nanji AA, Siakotos AN, Lin RC. Acetaldehyde-modified and 4-hydroxynonenal-modified proteins in the livers of rats with alcoholic liver disease. Hepatology. 1997;26:650-657. [PubMed] [DOI] |
| 26. | Lu X, Wang B, Xie Y, Liu C, Fu B. [Dynamic change and expression of matrix metalloproteinase-2, -9 in alcoholic liver disease in rats]. Zhonghua Gan Zang Bing Za Zhi. 2001;9:268-270. [PubMed] |
| 30. | Casini A, Ceni E, Salzano R, Milani S, Schuppan D, Surrenti C. Acetaldehyde regulates the gene expression of matrix-metalloproteinase-1 and -2 in human fat-storing cells. Life Sci. 1994;55:1311-1316. [PubMed] [DOI] |
| 31. | Zhu Y, Yin C, Ma X, Ma H, Jia J, Wang B. [The effect of herbal compound 861 on mRNA levels of MMP-2 and its activities in experimental rats liver fibrosis]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2002;16:348-350. [PubMed] |