修回日期: 2004-08-09
接受日期: 2004-08-30
在线出版日期: 2004-10-15
目的: 探讨经导管选择性门静脉栓塞(PVE)在肝癌治疗中的临床应用价值.
方法: 20例不能手术切除的晚期肝癌患者, 在电视透视引导下经导管行门静脉右支栓塞. 栓塞前、后用CT测量左侧肝叶的体积, 并测量栓塞前后的门静脉压力, 监测肝功能和凝血功能的变化.
结果: 20例患者均成功行门静脉右支栓塞, PVE术后左侧肝叶代偿增生明显, 13例(65%)在PVE后4 wk内左侧肝叶占整个肝脏的百分比达到或几乎达到了25%, 为手术切除创造了条件, 其中1例PVE后顺利实行右肝切除术. PVE后未出现门静脉高压, 肝功能损害轻, 均无并发症出现.
结论: 经导管选择性门静脉栓塞能诱导肝叶代偿性增生, 增加手术切除率, 提高手术切除的安全性, 对于大量无法手术切除的肝癌患者的治疗, 具有广泛的临床应用价值.
引文著录: 李建军, 杨维竹, 江娜, 黄兢姚, 郑曲彬, 黄宁, 杨升. 经导管选择性门静脉栓塞治疗肝癌的临床应用. 世界华人消化杂志 2004; 12(10): 2291-2294
Revised: August 9, 2004
Accepted: August 30, 2004
Published online: October 15, 2004
AIM: To evaluate the clinical value of transcatheter selective portal vein embolization (PVE) in treatment of hepatocellular carcinoma.
METHODS: Twenty patients, with unresectable advanced hepatocellular carcinoma, were treated with right PVE under fluoroscopic guidance. Left hepatic lobe volume was obtained by computerized tomography (CT) before and after PVE. Portal venous pressure, hepatic and thromboplastic functions were also detected before and after PVE.
RESULTS: Right portal vein were embolized successfully in 20 patients. Compensatory hypertrophy was observed in left hepatic lobe. The volume of left hepatic lobe increased significantly with a total percentage of 25% in 13 patients (65%) at 4 wk after PVE (P <0.01). Right hepatic lobe was successfully resected in 1 patient. No patients had complications such as portal hypertension after PVE. Slight damage of liver function after PVE was observed.
CONCLUSION: PVE can induce compensatory hypertrophy of liver lobes, which provides another operation chance for patients with unresectable hepatocellular carcinoma.
- Citation: Li JJ, Yang WZ, Jiang N, Huang JY, Zheng QB, Huang N, Yang S. Transcatheter selective portal vein embolization in treatment of hepatocellular carcinoma: an analysis of 20 cases. Shijie Huaren Xiaohua Zazhi 2004; 12(10): 2291-2294
- URL: https://www.wjgnet.com/1009-3079/full/v12/i10/2291.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i10.2291
选择性门静脉栓塞术在肝癌治疗中的应用国外报道较多[1-6], 其治疗原理主要是[7-13]: 选择性栓塞载瘤侧门静脉支, 使非栓塞侧肝叶代偿增生, 使因肿瘤体积较大、正常肝叶体积较小而不能行手术切除的患者获得切除的机会, 并可保证切除术后仍有足够的维持正常肝功能的肝脏存在, 从而避免因切除术后维持正常生理需要的肝脏体积较小而出现肝功能衰竭. 国内尚未见在电视透视引导下经导管选择性门静脉栓塞在肝癌治疗中的临床应用报道. 现报道如下.
不能手术的晚期肝癌患者20例, 原发性肝癌19例, 转移性肝癌(原发灶为小肠恶性间质瘤)1例; 男17例, 女3例, 年龄37-62(平均49.4±10.0岁); 肿瘤直径11-17(平均14.0±2.5 cm). 所选患者均满足以下几个条件: (1)肿瘤集中位于肝右叶; (2)肝功能为Child-Push A级; (3)无造影剂过敏史; (4)无出血倾向; (5)门静脉主干及左右支无瘤栓. 按肝癌的临床分型分期标准[14], 单纯型12例, 硬化型8例; 临床Ⅰ期16例, Ⅱ期4例.全部病例均经临床检查、甲胎蛋白(AFP)测定、CT、MRI、肝动脉造影或肝穿活检证实, 病理诊断1例, 临床诊断19例, 均符合全国肝癌协作会议制定的诊断标准.栓塞材料为明胶海绵、无水乙醇和不锈钢圈(美国COOK公司). 实验器械为 22G Chiba针和4.0 F血管扩张器(COOK), 0.018"微导丝、0.035"黑泥鳅导丝、5 F Cobra导管(日本TERUMO公司), 5 F血管鞘(爱尔兰AVE公司).
门静脉插管的途径: 经皮经肝门静脉插管和经皮经脾门静脉插管. 根据CT扫描(所有患者均在术前行CT增强扫描, 并作门静脉三维重建)及通过间接门静脉造影获得的门静脉系统情况, 确定穿刺点. Chiba针于剑突下偏左侧区域穿刺肝内门静脉左支或经左侧腋后线第7-9肋间穿刺脾静脉分支, 成功后用微导丝从Chiba针进至门静脉主干或肠系膜上静脉内, 然后将针退出, 沿导丝送入血管扩张器, 再通过黑泥鳅导丝交换送入血管鞘, 置入Cobra导管于门静脉主干造影; 将导管插入门静脉右支, 根据门静脉右支大小、血流速度等情况选用合适的栓塞剂, 将门静脉右支完全栓塞, 栓塞后再次造影, 证实门静脉右支完全闭塞.栓塞前后各测一次门静脉主干压力. 栓塞后均用明胶海绵、不锈钢圈将穿刺道栓塞以防止腹腔内出血. 经皮经肝门静脉插管法适用于门静脉左支肝内部分较粗大、易于穿刺, 且门静脉左右支成角为钝角, 通过左支易于将栓塞剂导入右支的患者.经皮经脾门静脉插管法适用于门静脉左支肝内部分较细小, 不宜直接穿刺或门静脉左右支成角为锐角, 通过左支不易将栓塞剂导入右支及脾脏较大的患者.所有患者均在PVE术前或术后配合肝动脉化疗栓塞术(transhepatic arterial chemotherapy embolization, TACE)治疗. 化疗药用5-氟脲嘧啶1 000 mg, 顺铂80 mg, 表阿酶素60 mg或丝裂酶素16 mg, 栓塞剂为超液态碘化油和明胶海绵条.
PVE前后门静脉造影以了解门静脉栓塞效果; 门静脉压力测定, 以了解栓塞前后压力变化情况; PVE前、PVE后2, 4, 8 wk行CT增强扫描, 并作门静脉三维重建, 用Volume功能分别测量整个肝脏(total hepatic lobe, THL)、右侧肝叶(right hepatic lobe, RHL)和左侧肝叶(left hepatic lobe, LHL)的体积, 以了解栓塞后左叶代偿增生情况及门脉右支是否再通; 行食管吞钡检查, 观察是否有食管静脉曲张, 以判断栓塞前后是否出现门静脉高压; PVE前、PVE后1, 3, 7 d检查肝功能、凝血功能以了解其在栓塞前后的变化情况; PVE后临床症状、体征的表现, 以观察PVE后是否有并发症出现.
统计学处理 对栓塞前后测定的左侧肝叶体积、左侧肝叶占整个肝脏的体积比(LHL/THL)、门静脉压力、ALT、AST、TBIL、PT通过SPSS11.5软件做方差分析, 门静脉压力采用配对t检验.
所有患者均在电视透视引导下成功实现门静脉插管及门静脉右支栓塞, 其中19例行经皮经肝门静脉插管栓塞, 1例行经皮经脾门静脉插管栓塞. 随访期间1例于PVE术后2 mo发现肺部转移, 术后3 mo死亡, 其余病例均存活. 存活病例中1例顺利实行右肝切除术, 随访10 mo未发现左肝内转移和远处转移, 2例分别于PVE术后3 mo和4 mo发现左肝内转移灶而未能行肿瘤切除, 剩余病例拒绝行手术切除治疗.
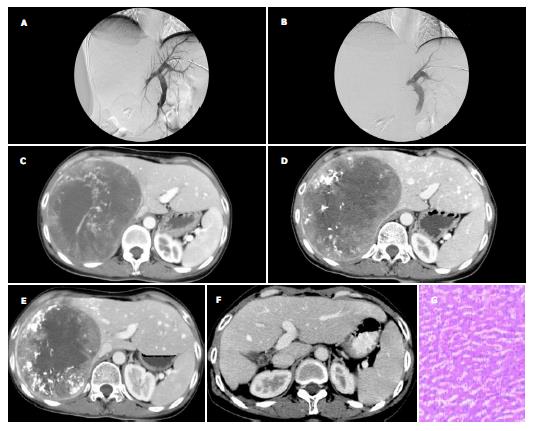
栓塞前门静脉主干及左右支血流均通畅, 呈树枝状逐级变细, 门静脉右支肿瘤周围分支部分受推压, 未见门静脉分支进入肿瘤内, 所有病例均未发现类似动脉造影所见的迂曲、紊乱的肿瘤血管网和肿瘤染色等征象; 经门静脉注入超液态碘化油, 未见碘化油在瘤内停聚.门静脉右支栓塞后, 门静脉右支完全闭塞, 呈截断表现, 门静脉主干及左支血流仍通畅; 所有病例均未发生栓塞剂反流致异位栓塞(图1A, B).CT扫描显示栓塞后门静脉右支完全闭塞, 未出现再通现象, 门静脉主干及左支血流仍通畅.
左侧肝叶体积术前为461.5±108.2 cm3, 术后2, 4, 8 wk分别为608.5±135.7 cm3, 660.2±133.8 cm3, 678.0±132.7 cm3, 分别比术前增加33.5±22.1%, 45.4±23.8%, 49.5±24.0%. 术前左侧肝叶占整个肝脏的体积百分比为18.4±5.1%, 术后2, 4, 8 wk分别为24.2±5.9%, 26.3±5.8%, 27.0±6.1%, 其中PVE后4 wk内左肝体积占整个肝脏体积百分比达到或几乎达到25%的有13例, 占总病例数的65%. 术后2 wk较术前体积增大显著(P <0.01), 术后4 wk与2 wk间、8 wk与2 wk间、8 wk与4 wk间体积增大均无显著性变化. 本组1例肝转移癌患者因无基础肝病, 无肝硬化, 肝脏代偿增生最明显, 其PVE术前左侧肝叶体积为396 cm3 , PVE后2, 4, 8 wk分别增至650 cm3, 780 cm3, 790 cm3, 为手术切除巨大右肝转移癌创造了良好的条件, PVE后3 mo行右肝切除术.切除术后未出现肝功能衰竭等并发症, PVE后5 mo体积增至1 395 cm3, PVE后7 mo体积增至1 420 cm3. 切除标本中左肝部分行病理检查示: 肝细胞增生、肥大, 可见许多双核肝细胞(图1C-G).
栓塞前门静脉压力为17.0±2.6 kPa, 栓塞后为19.6±2.8 kPa, 变化显著(P <0.01), 但均在正常值范围内(16-24 kPa), 未出现门静脉高压(>25 kPa). 栓塞术前钡透检查均显示食管黏膜光滑, 未见静脉曲张的表现, 术后均未出现食管静脉曲张.
PVE后1 d ALT, AST, TBIL明显升高(P <0.01), 但多数仍在正常值范围内, 3-7 d内逐渐恢复至术前水平; Child-Push评分均为A级. PVE后PT第1 d变化明显(P <0.01), 第3 d和第7 d无明显变化, 但均在正常值范围内(11-15 s). PVE后少数患者出现轻度腹痛、低热(不超过38 ℃)不适, 无需特别处理, 自行缓解; 无恶心、呕吐、皮肤黄、尿黄等不适. 所有患者PVE术后均未出现消化道出血、腹腔内出血、胆汁瘘、腹膜炎、腹水、肝功能衰竭、休克等严重并发症.
手术切除是治疗肝癌的一种主要手段, 临床上许多患者因肿瘤体积较大而失去手术机会, 手术切除率低, 约20-30%[15], 而且术后常会出现肝功能衰竭. 肝动脉化疗栓塞术(TACE)使晚期肝癌患者的预后有了很大进步, 部分患者获得了二期手术的机会, 取得了满意疗效[16]. 但二期手术率仅为8.4-28.2%[17]. 术前门静脉栓塞诱导肝叶代偿性增生可以扩大肝癌切除手术的适应证, 提高手术的安全性[18-19]. 肝叶增生的机制主要是: 门静脉分支栓塞后, 肝细胞分化和增生能力增强[20]; 门静脉血流重分配, 非栓塞侧血流增加[21], 而门静脉中含有促进肝细胞再生的营养物质[22]; 门静脉血流变化可促使IL-6从内皮细胞释放, 进而促进非栓塞侧肝叶增生[23]. 我们在电视透视引导下, 经导管行经皮经肝或经皮经脾门静脉栓塞的方法, 对20例肝癌患者成功实现门静脉右支栓塞, 成功率100%. 栓塞后左侧肝叶代偿性增生, 体积较栓塞前显著增大. 对于切除术后能维持正常肝功能的残余肝脏体积最小比例, 目前没有一致的观点[4,7,24-25]. 本组有13例(65%)患者在PVE术后4 wk内左侧肝叶占整个肝脏的百分比达到或几乎达到了25%, 为手术切除创造了条件.1例患者行门静脉右支栓塞后, 左叶代偿增生明显, 术后行右肝手术切除, 未出现肝功能衰竭等并发症, 切除术后肝脏继续代偿增生.术后均无并发症出现, 少数患者出现轻度腹痛、低热不适, 可自行缓解.肝功能、凝血功能在1 wk内恢复至术前水平, Child-Push评分术前、后均为A级, 而且肝脏体积增大常伴随着肝功能的增强[26-29]. 门静脉压力栓塞前后有显著性变化, 但多数仍在正常值范围内, 未出现门静脉高压. 经导管门静脉栓塞诱导肝叶代偿性增生在临床上的应用也是安全的.
可用于门静脉栓塞的栓塞剂的品种很多, 常用氰基丙烯酸正辛酯、碘油、明胶海绵、凝血酶、无水乙醇、不锈钢圈、PVA颗粒、纤维蛋白胶、Embol-78等, 也有人混合使用上述材料. 理想的栓塞剂应是门静脉末梢与近端均永久性栓塞, 而不发生再通;安全、损伤轻、患者可忍受; 容易输送; 价格便宜. 我们采用明胶海绵、无水乙醇、不锈钢圈三种价格便宜的物质联合栓塞取得了很好的效果.
总之, 门静脉右支栓塞确能诱导左侧肝叶代偿性增生, 可增加手术切除率, 提高手术切除的安全性, 对于大量无法手术切除的肝癌患者的治疗, 具有广泛的临床应用价值.选择性门静脉栓塞术应作为每一个外科医生治疗肝癌的一种选择.
| 1. | Wakabayashi H, Ishimura K, Okano K, Karasawa Y, Goda F, Maeba T, Maeta H. Application of preoperative portal vein embolization before major hepatic resection in patients with normal or abnormal liver parenchyma. Surgery. 2002;131:26-33. [PubMed] [DOI] |
| 2. | Madoff DC, Hicks ME, Vauthey JN, Charnsangavej C, Morello FA Jr, Ahrar K, Wallace MJ, Gupta S. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics. 2002;22:1063-1076. [PubMed] [DOI] |
| 3. | Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-680; discussion 680-681. [PubMed] [DOI] |
| 4. | Elias D, Ouellet JF, De Baère T, Lasser P, Roche A. Preoperative selective portal vein embolization before hepatectomy for liver metastases: long-term results and impact on survival. Surgery. 2002;131:294-299. [PubMed] [DOI] |
| 5. | Ko GY, Sung KB, Yoon HK, Kim JH, Weon YC, Song HY. Preoperative portal vein embolization with a new liquid embolic agent. Radiology. 2003;227:407-413. [PubMed] [DOI] |
| 6. | Doros A, Weszelits V, Puhl M, Fehérvári I, Alföldy F. [Percutaneous portal vein embolization before major hepatic resection]. Magy Seb. 2003;56:39-44. [PubMed] |
| 7. | Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, Hawkins IF, Vauthey JN. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686-691; discussion 691-693. [PubMed] [DOI] |
| 8. | Jaeck D, Bachellier P, Nakano H, Oussoultzoglou E, Weber JC, Wolf P, Greget M. One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg. 2003;185:221-229. [PubMed] [DOI] |
| 9. | Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766-774. [PubMed] [DOI] |
| 10. | Kim MJ, Choo SW, Do YS, Park KB, Han YH, Choo IW, Cho JM, Cho JW, Kim SJ, Sohn TS. Use of double-occlusion balloon catheter: preoperative portal vein embolization for induction of future remnant liver hypertrophy. Cardiovasc Intervent Radiol. 2004;27:16-20. [PubMed] [DOI] |
| 11. | Adam R, Lucidi V, Bismuth H. Hepatic colorectal metastases: methods of improving resectability. Surg Clin North Am. 2004;84:659-671. [PubMed] [DOI] |
| 12. | Kokudo N, Makuuchi M. Current role of portal vein embolization/hepatic artery chemoembolization. Surg Clin North Am. 2004;84:643-657. [PubMed] [DOI] |
| 13. | Shimada H, Tanaka K, Masui H, Nagano Y, Matsuo K, Kijima M, Ichikawa Y, Ike H, Ooki S, Togo S. Results of surgical treatment for multiple (> or = 5 nodules) bi-lobar hepatic metastases from colorectal cancer. Langenbecks Arch Surg. 2004;389:114-121. [PubMed] [DOI] |
| 16. | Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou XD, Zhou J, Qiu SJ, Lu JZ. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998;15:674-678. [PubMed] [DOI] |
| 18. | Lu MD, Chen JW, Xie XY, Liang LJ, Huang JF. Portal vein embolization by fine needle ethanol injection: experimental and clinical studies. World J Gastroenterol. 1999;5:506-510. [PubMed] [DOI] |
| 19. | Ji W, Li JS, Li LT, Liu WH, Ma KS, Wang XT, He ZP, Dong JH. Role of preoperative selective portal vein embolization in two-step curative hepatectomy for hepatocellular carcinoma. World J Gastroenterol. 2003;9:1702-1706. [PubMed] [DOI] |
| 20. | Harada H, Imamura H, Miyagawa S, Kawasaki S. Fate of the human liver after hemihepatic portal vein embolization: cell kinetic and morphometric study. Hepatology. 1997;26:1162-1170. [PubMed] |
| 21. | Wakabayashi H, Nakano S, Ishimura K, Hagiike M, Okano K, Maeba T, Maeta H. Changes in arterial and portal perfusion in embolized and nonembolized hepatic lobes after portal vein embolization evaluated by helical computed tomography. Surg Today. 2001;31:991-995. [PubMed] [DOI] |
| 22. | Starzl TE, Francavilla A, Halgrimson CG, Francavilla FR, Porter KA, Brown TH, Putnam CW. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:179-199. [PubMed] |
| 23. | Kawai M, Naruse K, Komatsu S, Kobayashi S, Nagino M, Nimura Y, Sokabe M. Mechanical stress-dependent secretion of interleukin 6 by endothelial cells after portal vein embolization: clinical and experimental studies. J Hepatol. 2002;37:240-246. [PubMed] [DOI] |
| 24. | Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness--study in 26 patients. Radiology. 2003;227:251-260. [PubMed] [DOI] |
| 25. | Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519. [PubMed] [DOI] |
| 26. | Sugai Y, Komatani A, Hosoya T, Yamaguchi K. Response to percutaneous transhepatic portal embolization: new proposed parameters by 99mTc-GSA SPECT and their usefulness in prognostic estimation after hepatectomy. J Nucl Med. 2000;41:421-425. [PubMed] |
| 27. | Chijiiwa K, Saiki S, Noshiro H, Kameoka N, Nakano K, Tanaka M. Effect of preoperative portal vein embolization on liver volume and hepatic energy status of the nonembolized liver lobe in humans. Eur Surg Res. 2000;32:94-99. [PubMed] [DOI] |