修回日期: 2003-03-16
接受日期: 2003-03-28
在线出版日期: 2003-09-15
观察中药复方胃肠安诱导肝癌细胞分化的作用.
以SMMC-7721人肝癌细胞为研究对象, 维甲酸为对照, 采用药物血清添加法, 通过MTT法和Alamar Blue法观察胃肠安对肝癌细胞增生的抑制作用; 放射免疫法观察对肝癌细胞分泌甲胎蛋白和白蛋白的影响; Western blot观察对肝癌细胞P53、P16以及P21蛋白表达的影响.
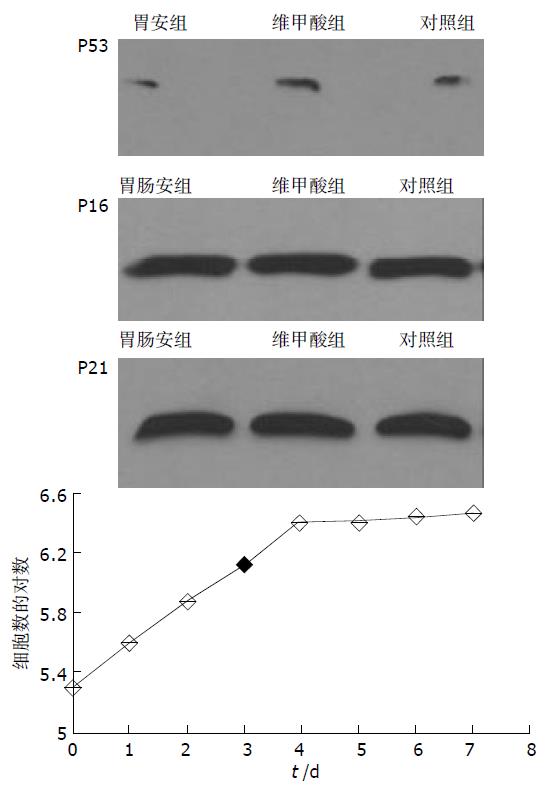
MTT法显示胃肠安与维甲酸对SMMC-7721人肝癌细胞增生均有抑制作用, 胃肠安在48 h达到最大, 而维甲酸对细胞增生的抑制作用在48 h后逐渐下降, 较胃肠安组明显降低. Alamar Blue法结果显示, 16 h后, 胃肠安组细胞还原的Alamar Blue值较对照组明显减少, 并在32 h后较维甲酸组显著下降, 提示与维甲酸相比, 胃肠安作用起效慢但持续时间长; 胃肠安组分泌的甲胎蛋白较对照组显著减少(11.4±1.4 mg/L vs 17.2±1.1 mg/L, P =0.036), 而白蛋白显著增多(0.40±0.02 mg/L vs 0.29±0.01 mg/L, P =0.043); 胃肠安组突变型P53蛋白的表达较对照组明显减少, 而P16蛋白和P21蛋白的表达较对照组增多.
胃肠安方剂具有抑制SMMC-7721人肝癌细胞增生、诱导细胞分化的作用, 其机制可能在于减少突变型P53蛋白表达和增加P16, P21蛋白表达.
引文著录: 赵海磊, 刘成, 赵爱光. 中药复方胃肠安血清诱导肝癌SMMC-7721细胞分化. 世界华人消化杂志 2003; 11(9): 1345-1348
Revised: March 16, 2003
Accepted: March 28, 2003
Published online: September 15, 2003
To observe the differentiation in hepatocellular carcinoma cell line induced by Chinese medicine recipe Weichangan.
Weichangan, contrasted by the retinoic acid and distilled water, was made by using serum pharmacological method. The inhibition on the growth of SMMC-7721 cell line by Weichangan was observed through the method of MTT and Alamar Blue. Radioimmunoassay was applied to determine the concentration of a-fetoprotein and albumin secreted by the incubated cells. Western blot method was used to detect the mutant p53, p16 and p21 protein expression in SMMC-7721 cell line.
MTT assay showed both Weichangan and serum retinoic acid had inhibiting effect on the proliferation of human hepatocellular carcinoma SMMC-7721 cell line. Weichangan reached its maximal inhibition effect after 48 hours, while the effect of retinoic acid decreased gradually after 48 hours. Alamar Blue method showed that significant decrease was found in serum Weihangan after 16 hours compared with that in the control. After 32 hours, the decrease induced by Alamar Blue was more significant than that in cells incubated with serum Weichangan compared with those incubated in serum retinoic acid, indicating the gradual and durable action of Weichangan recipe. The decreased amount (11.4±1.4 mg/L vs 17.2±1.1 mg/L, P =0.036) of a-fetoprotein and increased amount (0.40±0.02 mg/L vs 0.29±0.01 mg/L, P =0.043) of albumin were found in the cells incubated in serum Weichangan. Western blot method showed decreased expression of p53 protein and increased expression of p16 and p21 protein in cells incubated in serum Weichangan.
The results suggest that Weichangan inhibits the growth of SMMC-7721 cell line and induces the differentiation in this hepatocellular carcinoma cell line. The effect on p53, p16 and p21 may be the mechanisms of Weichangan in inducing the differentiation of this cell lines.
- Citation: Zhao HL, Liu C, Zhao AG. Differentiation of hepatocellular carcinoma SMMC-7721 cell line induced by Chinese medicine recipe Weichangan. Shijie Huaren Xiaohua Zazhi 2003; 11(9): 1345-1348
- URL: https://www.wjgnet.com/1009-3079/full/v11/i9/1345.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i9.1345
癌症是分化异常的疾病[1-3], 分化障碍可能决定了肿瘤细胞的恶性程度[4, 5], 因此诱导分化已成为肿瘤治疗学研究的热点[6,7]. 中药复方胃肠安以健脾扶正中药为主, 临床与实验研究证实对包括肝癌在内的消化道肿瘤有良好的防治效果. 我们重点研究胃肠安方剂防治肝癌的机制是否在于诱导肝癌细胞分化.
选用人肝癌细胞株SMMC-7721, 购自中国科学院细胞生物学研究所. Wistar大鼠, 质量250±30 g, 普通级, 常规颗粒饲料喂养, 由上海中医药大学实验动物中心提供, 制备药物血清用. 胃肠安(太子参12 g、炒白术12 g、茯苓30 g、姜半夏9 g、红藤30 g、野葡萄藤30 g、藤梨根30 g、陈皮4.5 g、生牡蛎30 g、夏枯草9 g、绿萼梅9 g、青皮4.5 g), 配制成 100 g/L的灌胃液. 全反式维甲酸由瑞金医院血液研究所提供, 配制成0.5 g/L的灌胃液. RPMI1640培养基干粉和胎牛血清(FBS), Gibco公司产品; 噻唑蓝 (MTT)购自华美生物制品有限公司; Alamar Blue: Nalgene Biosource公司产品; 甲胎蛋白与白蛋白放免分析药盒, 上海生物制品研究所产品; 兔抗P53多克隆抗体, 博士德生物科技有限公司产品; p21WAF1/Cip1/Sdi1/Pic1鼠单克隆抗体、p16INK4a/MTS1鼠单克隆抗体, Antibody Diagnostica Inc.产品; 抗鼠Ig、抗兔Ig, Amersham Pharmacia Biotech公司产品.
SMMC-7721细胞以含100 mL/L FBS的RPMI1640培养液, 37 °C, 50 mL/L CO2培养96 h. 细胞传代以2.5 g/L Trypsin-EDTA消化细胞后, 加入FBS中止消化. 药物血清制备按参考文献[8]进行. 大鼠分别以20 mL/kg灌以胃肠安灌胃液与维甲酸灌胃液(均相当于临床成人用量的10倍), 对照组灌以等量生理盐水, 共3 d, 2次/d, 2次灌胃间隔6 h. 第3 d灌胃结束后禁食, 次日晨再灌胃1次. 1 h后下腔静脉取血. 每只大鼠取5-8 mL静脉血, 离心分离血清, 即为胃肠安药物血清、维甲酸药物血清和对照血清. 56 °C水浴灭活30 min后分装, 置低温冰箱中备用. 随机分为胃肠安组、维甲酸组、对照组, 分别添加9:1的RPMI1640/胃肠安药物血清、RPMI1640/维甲酸药物血清、RPMI1640/对照血清, 使配制的各组细胞温育液中药物血清浓度为100 mL/L.
1.2.1 SMMC-7721细胞增生检测 采用MTT法[9]: 各孔加入细胞温育液100 uL培养12, 24, 48, 72, 96 h观察药物血清对细胞增生的抑制作用. Alamar Blue法[10]. SMMC-7721细胞以5.0×103/孔加入96孔板. 细胞贴壁后加入各组细胞温育液和Alamar Blue, Alamar Blue在总体积中浓度为100 mL/L. 培养0, 8, 16, 24, 32, 40, 48, 72 h后酶标仪570 nm和590 nm分别测A值, 计算AB值.
1.2.2 甲胎蛋白及白蛋白含量的测定 SMMC-7721细胞以2.5×108/L种于培养瓶中. 细胞贴壁后分别加入各组细胞温育液2 ml. 48 h后吸弃上清, 加入RPMI1640培养液2 mL培养48 h. 吸取上清液于离心管中, 1 000 g, 离心5 min. 取上清1.5 mL冷冻干燥. 采用放免法测定.
1.2.3 P53, P16和P21蛋白的表达 采用Western blot方法. 蛋白样品制备: SMMC-7721细胞以2.5108/L接种于培养皿. 贴壁后分别加入各组细胞温育液. 48 h后加入RIPA裂解液150 mL将贴壁细胞刷下, 移入离心管内. 4 °C, 15 000 g离心15 min, 取上清, -20 °C保存. 取4 mL标准品或样品分别与考马斯亮蓝溶液2 mL混匀, 595 nm波长处测吸光度. 计算每组样品浓度, 确定上样量. 电泳: 灌制分离胶和积层胶. 按以上计算结果平衡上样量, 余数用裂解液补齐. 95 °C水浴5 min. 倒入电泳缓冲液. 加样品和Marker, 电压90 V, 电泳120 min. 转膜: 剪取硝酸纤维素滤膜贴于凝胶上, 并在转膜装置中放置冰盒. 加转移缓冲液. 电流0.22 mA, 转膜90 min. 杂交与检测: 滤膜以50 g/L脱脂奶粉的TBS封闭抗原. 加入兔抗P53多克隆抗体, 稀释度1:200, 4 °C, 摇床摇动过夜. TTBS洗3次. 加入抗兔Ig, 稀释度1:2 000, 室温下摇动1h. TTBS洗3次. 将滤膜浸入ECL中, 1 min后取出, 暗匣内固定. 暗室中放入胶片. 曝光15 min, 显影2 min, 清水洗后再定影5 min, 观察实验结果. 以b-巯基乙醇洗膜后, 一抗加p21WAF1/Cip1/Sdi1/Pic1或p16INK4a/MTS1鼠单克隆抗体进行P21和P16蛋白的观察, 二抗为抗鼠Ig.
统计学处理 采用双侧t 检验.
MTT分析结果显示胃肠安及维甲酸药物血清对该细胞增生有抑制作用(表1). 培养16 h后, 胃肠安组还原的Alamar Blue值与对照组相比明显减少(P <0.05); 维甲酸组在16, 24 h与对照组相比有显著差异, 但随后的观察点未见显著差异; 胃肠安组在32, 40, 48, 72 h与维甲酸组相比有显著差异(P <0.05, 表2).
胃肠安组甲胎蛋白分泌量较对照组明显减少(11.4±1.4 mg/L vs 17.2±1.1 mg/L, P =0.036), 白蛋白分泌量较对照组明显增多(0.40±0.02 mg/L vs 0.29±0.01 mg/L, P =0.043); 维甲酸组甲胎蛋白量与白蛋白量较对照组也有减少与增多的趋势, 但统计未见明显差异.
胃肠安组突变型P53蛋白表达较对照组和维甲酸组明显减少. 密度扫描积分: 胃肠安组 3 559, 维甲酸组 4 277, 对照组 4 042; 胃肠安组P16蛋白表达较维甲酸组和对照组增多. 密度扫描积分: 胃肠安组 5 265, 维甲酸组 4 992, 对照组 4 797; 维甲酸组和胃肠安组P21蛋白表达较对照组增多. 密度扫描积分: 胃肠安组 6 171, 维甲酸组 6 764, 对照组 5 809.
SMMC-7721人肝癌细胞是目前体外研究肝癌常用的细胞株[11-13]. 我们在细胞传代后24h添加药物血清, 作用48 h后观察实验结果. 全反式维甲酸具有诱导Bel-7402和SMMC-7721人肝癌细胞分化的作用[14,15], 因而本研究中以维甲酸为对照. MTT法显示胃肠安及维甲酸药物血清对SMMC-7721细胞增生有一定的抑制作用, 维甲酸最大抑制作用出现在24 h和48 h, 而胃肠安出现在48 h和72 h. Alamar Blue为一种染料, 在细胞培养上清液中可作为氧分子电子传递链的受体, 因而可以反映所研究的细胞对氧分子的消耗, 以观察细胞的代谢状况[16-19]. 与MTT分析法相比, Alamar Blue法可以连续、快速地检测细胞的增生状态, 并且由于是对同一批细胞的增生状态进行观察, 因此有操作简便和几乎不干扰细胞正常代谢的特点. 结果显示胃肠安组细胞代谢状态最低, 表明胃肠安对该细胞增生具有抑制作用; 维甲酸组在16、24 h与对照组相比有差异, 但24 h后观察未见显著差异, 说明维甲酸抑制细胞增生的作用减弱. 综合MTT与Alamar Blue的结果, 胃肠安对SMMC-7721肝癌细胞增生有抑制作用. 与维甲酸相比, 其抑制作用起效慢, 但持续时间长. 甲胎蛋白和白蛋白是目前较为公认的反映肝癌细胞分化程度的指标, 甲胎蛋白是肝癌细胞去分化的标志, 而白蛋白是分化的标志. 实验结果显示胃肠安组细胞分泌的甲胎蛋白量较对照组明显减少, 而分泌的白蛋白显著增多, 提示胃肠安对该肝癌细胞具有诱导分化作用.
P53过表达参与肝癌去分化和增生, 有研究显示p53基因突变与肝癌的分化等级、肝癌微血管侵犯显著相关, 可导致肿瘤早期转移, 是肝癌预后差的指标 [20-28]. 本结果显示胃肠安组中突变型P53蛋白表达较对照组明显减少, 提示胃肠安方剂有降低SMMC-7721人肝癌细胞突变型P53蛋白表达的作用. P16蛋白通过直接作用于CDK4/6, 阻滞其与CyclinD结合, 避免Rb蛋白磷酸化及E2F因子释放, 将细胞周期停滞在G1期, 从而抑制细胞过度增生. 已发现P16蛋白表达缺失与肝癌分化密切相关[29-36]. 我们观察到胃肠安组细胞中P16蛋白表达较对照组增多, 提示胃肠安可能通过增加P16蛋白表达诱导该肿瘤细胞分化. p21是调节细胞生长和分化的重要基因, 几乎能抑制与Rb基因磷酸化有关的所有周期素-周期素依赖激酶复合物[37-40]. 实验结果胃肠安组细胞中P21蛋白表达较对照组增多, 提示胃肠安方剂可能通过增加P21蛋白表达抑制细胞增生, 诱导细胞分化. 实验中观察到维甲酸组P21蛋白表达明显增多, 可能与维甲酸通过该作用环节诱导肝癌细胞分化有关.
分化是组织胚胎学概念. 细胞正常分化过程受到基因的严格调控. 本实验观察到中药复方胃肠安血清减少SMMC-7721人肝癌细胞突变型P53蛋白表达和增加P16、P21蛋白表达, 这可能是胃肠安诱导该肝癌细胞分化的分子生物学机制.
| 1. | Kanazawa T, Watanabe T, Kazama S, Tada T, Koketsu S, Nagawa H. Poorly differentiated adenocarcinoma and mucinous carcinoma of the colon and rectum show higher rates of loss of heterozygosity and loss of E-cadherin expression due to methylation of promoter region. Intern J Cancer. 2002;102:225-229. [DOI] |
| 2. | Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, Yang JM. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8:987-993. [DOI] |
| 3. | Yamamoto H, Ochiya T, Takeshita F, Toriyama-Baba H, Hirai K, Sasaki H, Sasaki H, Sakamoto H, Yoshida T, Saito I. Enhanced skin carcinogenesis in cyclin D1-conditional transgenic mice: cyclin D1 alters keratinocyte response to calcium-induced terminal differentiation. Cancer Research. 2002;62:1641-1647. |
| 4. | Faderl S, Keating MJ, Do KA, Liang SY, Kantarjian HM, O'Brien S, Garcia-Manero G, Manshouri T, Albitar M. Expression profile of 11 proteins and their prognostic significance in patients with chronic lymphocytic leukemia (CLL). Leukemia. 2002;16:1045-1052. [DOI] |
| 5. | Schmied BM, Ulrich AB, Matsuzaki H, El-Metwally TH, Ding X, Fernandes ME, Adrian TE, Chaney WG, Batra SK, Pour PM. Biologic instability of pancreatic cancer xenografts in the nude mouse. Carcinogenesis. 2000;21:1121-1127. [DOI] |
| 6. | Charrad RS, Gadhoum Z, Qi J, Glachant A, Allouche M, Jasmin C, Chomienne C, Smadja-Joffe F. Effects of anti-CD44 monoclonal antibodies on differentiation and apoptosis of human myeloid leukemia cell lines. Blood. 2002;99:290-299. [DOI] |
| 7. | Chambery D, De Galle B, Ehrenborg E, Babajko S. Multi-hormonal regulation of IGFBP-6 expression in human neuroblastoma cells. Growth Horm IGF Res. 2000;10:349-359. [DOI] |
| 8. | Liu P, Zhou JF, Hu YY, Liu CH, Lin C. Effect and significance of "benefiting qi and nourishing yin" on induction of hepatoma cell differentiation. Zhongguo Zhongyi Jichu Yixue Zazhi. 2000;6:29-34. |
| 9. | Mao H, Zhang L, Wang Y, Li X. Experimental studies of icariin on anticancer mechanism. Zhongyaocai. 2000;23:554-556. |
| 10. | Elattar TM, Virji AS. Effect of tea polyphenols on growth of oral squamous carcinoma cells in vitro. Anticancer Res. 2000;20:3459-3465. |
| 11. | Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of Nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483-487. [DOI] |
| 12. | Chan RC, Xie H, Zhao GP, Xie Y. Dendritomas formed by fusion of mature dendritic cells with allogenic human hepatocellular carcinoma cells activate autologous cytotoxic T lymphocytes. Immunol Letters. 2002;83:101-109. [DOI] |
| 13. | Fang Y, Jin JW, Zha XL. Role of FAK in TNF-alpha/Cycloheximide-induced apoptosis of SMMC-7721 cells. Shengwu Huaxue Yu Shengwu Wuli Xuebao. 2001;33:53-58. |
| 14. | Wang L, Tang Z, Xue Q, Sun H, Chen J, Gao D, Zhao Y, Chen J, Sun R, Liu Y. Effects of interferon-alpha on recurrence and metastasis of hepatocellular carcinoma after curative resection in nude mice. Zhonghua Ganzangbing Zazhi. 2001;9:154-156. |
| 15. | Chen HM, Huang W, Huang JQ. Effect of retinoic acid and sodium butyrate on expression of hepatocarcinoma specific enzymes. J Pract Oncol. 2000;15:11-13. |
| 16. | DeForge LE, Billeci KL, Kramer SM. Effect of IFN-gamma on the killing of S. aureus in human whole blood. Assessment of bacterial viability by CFU determination and by a new Method using alamarBlue. J Immunol Methods. 2000;245:79-89. [DOI] |
| 17. | Back SA, Khan R, Gan X, Rosenberg PA, Volpe JJ. A new Alamar Blue viability assay to rapidly quantify oligodendrocyte death. J Neurosci Methods. 1999;91:47-54. [DOI] |
| 18. | Mountzouros KT, Howell AP. Detection of complement-mediated antibody-dependent bactericidal activity in a fluorescence-based serum bactericidal assay for group B Neisseria meningitidis. J Clin Microbiol. 2000;38:2878-2884. |
| 19. | Gloeckner H, Jonuleit T, Lemke HD. Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar BlueTM. J Immunol Methods. 2001;252:131-138. [DOI] |
| 20. | Liu H, Wang Y, Zhou Q, Gui SY, Li X. The point mutation of p53 gene exon7 in hepatocellular carcinoma from Anhui Province, a non HCC prevalent area in China. World J Gastroenterol. 2002;8:480-482. [DOI] |
| 21. | Qin LX, Tang ZY, Ma ZC, Wu ZQ, Zhou XD, Ye QH, Ji Y, Huang LW, Jia HL, Sun HC. P53 immunohistochemical scoring: an independent prognostic marker for patients after hepatocellular carcinoma resection. World J Gastroenterol. 2002;8:459-463. [DOI] |
| 22. | Martins C, Kedda MA, Kew MC. Characterization of six tumor suppressor genes and microsatellite instability in hepatocellular carcinoma in southern African blacks. World J Gastroenterol. 1999;5:470-476. [DOI] |
| 23. | Peng XM, Peng WW, Yao JL. Codon 249 mutations of p53 gene in development of hepatocellular carcinoma. World J Gastroenterol. 1998;4:125-127. [DOI] |
| 24. | Ming L, Yuan B, Thorgeirsson SS. Characteristics of high frequency 249 codon mutation of p53 gene in hepatocellular carcinoma in prevalent area of China. Zhonghua Zhongliu Zazhi. 1999;21:122-124. |
| 25. | Shao J, Li Y, Li H, Wu Q, Hou J, Liew C. Deletion of chromosomes 9p and 17 associated with abnormal expression of p53, p16/MTS1 and p15/MTS2 gene protein in hepatocellular carcinomas. Chin Med J (Engl). 2000;113:817-822. |
| 26. | Zhu Z, Zhu M, Ni C. Significance of p33(ING1b) and p53 gene expression in hepatocellular carcinoma. Zhonghua Yixue Zazhi. 2002;82:1332-1336. |
| 27. | Ming L, Thorgeirsson SS, Gail MH, Lu P, Harris CC, Wang N, Shao Y, Wu Z, Liu G, Wang X. Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology. 2002;36:1214-1220. [DOI] |
| 28. | Niu ZS, Li BK, Wang M. Expression of p53 and C-myc genes and its clinical relevance in the hepatocellular carcinomatous and pericarcinomatous tissues. World J Gastroenterol. 2002;8:822-826. [DOI] |
| 29. | Liew CT, Li HM, Lo KW, Leow CK, Chan JY, Hin LY, Lan WY, Lai PB, Lim BK, Huang J. High frequency of p16INK4A gene alterations in hepatocellular carcinoma. Oncogene. 1999;18:789-795. |
| 30. | Liew CT, Li HM, Lo KW, Leow CK, Lau WY, Hin LY, Lim BK, Lai PB, Chan JY, Wang XQ. Frequent allelic loss on chromosome 9 in hepatocellular carcinoma. Int J Cancer. 1999;81:319-324. |
| 31. | Luo D, Liu Q, Su J. The expression of p16, CDK4 and pRb in hepatocellular carcinomas. Zhonghua Ganzangbing Zazhi. 1999;7:94-95. |
| 32. | Jin M, Piao Z, Kim NG, Park C, Shin EC, Park JH, Jung HJ, Kim CG, Kim H. P16 is a major inactivation target in hepatocellular carcinoma. Cancer. 2000;89:60-68. [DOI] |
| 33. | Suh SI, Pyun HY, Cho JW, Baek WK, Park JB, Kwon T, Park JW, Suh MH, Carson DA. 5-Aza-2-deoxycytidine leads to down-regulation of aberrant p16INK4A RNA transcripts and restores the functional retinoblastoma protein pathway in hepatocellular carcinoma cell lines. Cancer Letters. 2000;160:81-88. [DOI] |
| 34. | Saito Y, Kanai Y, Sakamoto M, Saito H, Ishii H, Hirohashi S. Expression of mRNA for DNA methyltransferases and methyl-CpG-binding proteins and DNA methylation status on CpG islands and pericentromeric satellite regions during human hepatocarcinogenesis. Hepatology. 2001;33:561-568. |
| 35. | Azechi H, Nishida N, Fukuda Y, Nishimura T, Minata M, Katssuma H, Kuno M, Ito T, Komeda T, Kita R. Disruption of the p16/cyclin D1/retinoblastoma protein pathway in the majority of human hepatocellular carcinomas. Oncology. 2001;60:346-354. |
| 36. | Huang J, Shen W, Li B, Luo Y, Liao S, Zhang W, Cheng N. Molecular and immunohistochemical study of the inactivation of the p16 gene in primary hepatocellular carcinoma. Chin Med J (Engl). 2000;113:889-893. |
| 37. | Shi YZ, Hui AM, Takayama T, Li X, Cui X, Makuuchi M. Reduced p21(WAF1/CIP1) protein expression is predominantly related to altered p53 in hepatocellular carcinomas. British J Cancer. 2000;83:50-55. |
| 38. | Gong Y, Deng S, Zhang M, Wang G, Minuk GY, Burczynski F. A cyclin-dependent kinase inhibitor (p21(WAF1/CIP1)) affects thymidine incorporation in human liver cancer cells. British J Cancer. 2002;86:625-629. [DOI] |
| 39. | Yoshizawa K, Cioca DP, Kawa S, Tanaka E, Kiyosawa K. Peroxisome proliferator-activated receptor gamma ligand troglitazone induces cell cycle arrest and apoptosis of hepatocellular carcinoma cell lines. Cancer. 2002;95:2243-2251. [DOI] |
| 40. | Suzui M, Masuda M, Lim JT, Albanese C, Pestell RG, Weinstein IB. Growth inhibition of human hepatoma cells by acyclic retinoid is associated with induction of p21 (CIP1) and inhibition of expression of cyclin D1. Cancer Research. 2002;62:3997-4006. |