Copyright
©The Author(s) 2003. Published by Baishideng Publishing Group Inc. All rights reserved.
胃黏膜癌变过程中PTEN基因编码产物的表达及意义
李异玲, 何向民, 郑华川, 吴东瑛, 杨雪飞, 辛彦, 傅宝玉
李异玲, 何向民, 傅宝玉, 中国医科大学附属第一医院消化内科 辽宁省沈阳市 110001
李异玲, 女, 1972-09-29生, 辽宁省沈阳市人, 汉族, 博士生, 讲师, 中国医科大学附属第一医院消化内科.
郑华川, 吴东瑛, 杨雪飞, 辛彦, 中国医科大学附属第一医院肿瘤研究所, 第四研究室. 辽宁省沈阳市 110001
ORCID number: $[AuthorORCIDs]
基金项目: 国家自然科学基金资助课题, No. 30070845
通讯作者: 辛彦, 110001, 辽宁省沈阳市, 中国医科大学附属第一医院肿瘤研究所, 第四研究室. yxin@mail.cmu.edu.cn
电话: 024-23256666-6351
收稿日期: 2003-03-06
修回日期: 2003-03-12
接受日期: 2003-03-25
在线出版日期: 2003-09-15
目的
观察抑癌基因PTEN编码产物在胃黏膜癌变过程中的表达, 探讨PTEN表达与胃癌发生的关系.
方法
选取胃镜下正常胃黏膜、慢性浅表性胃炎、萎缩性胃炎无肠化、萎缩性胃炎伴肠化、中度不典型增生、重度不典型增生标本各60例, 选取手术后早期胃癌、进展期胃癌标本各60例, 应用S-P免疫组化方法检测各种胃黏膜病变中PTEN编码产物表达, 比较其表达与胃癌发生的关系.
结果
PTEN编码产物在正常胃黏膜、慢性浅表性胃炎、无肠化萎缩性胃炎、伴肠化萎缩性胃炎、中度异型增生、重度异型增生、早期胃癌和进展期胃癌中的阳性表达率分别为100%, 98.3%, 91.6%, 78.3%, 75%, 63.3%, 61.7%, 43.3%. 在检测的120例胃癌中, 肠型胃癌76例, PTEN表达率为60.5%, 弥漫型胃癌44例, PTEN表达率为38.6%.
结论
PTEN基因编码蛋白在胃癌发生过程中进行性下调, PTEN蛋白表达可作为判定胃癌生物学行为的客观指标.
关键词: N/A
引文著录: 李异玲, 何向民, 郑华川, 吴东瑛, 杨雪飞, 辛彦, 傅宝玉. 胃黏膜癌变过程中PTEN基因编码产物的表达及意义. 世界华人消化杂志 2003; 11(9): 1294-1296
Expression of PTEN encoding product in malignant lesions of gastric mucosa and its significance
Yi-Ling Li, Xiang-Min He, Hua-Chuan Zheng, Dong-Ying Wu, Xue-Fei Yang, Yan Xin, Bao-Yu Fu
Yi-Ling Li, Xiang-Min He, Bao-Yu Fu, Department of Digestive diseases, The First Affiliated Hospital of China Medical University, Nanjing North Street 155, Shenyang 110001, Liaoning Province, China
Hua-Chuan Zheng, Dong-Ying Wu, Xue-Fei Yang, Yan Xin, Cancer Institute, The First Affiliated Hospital of China Medical University, Nanjing North Street 155, Shenyang 110001, China
Correspondence to: Dr. Yan Xin, Department of Digestive Disease, The First Affiliated Hospital of China Medical University, Nanjing North Street 155, Shenyang 110001, Liaoning Province, China. yxin@mail.cmu.edu.cn
Received: March 6, 2003
Revised: March 12, 2003
Accepted: March 25, 2003
Published online: September 15, 2003
AIM
To observe the expression of PTEN protein in gastric cancer and precancerous lesions, and to investigate the relationship between PTEN expression and the pathogenesis of gastric cancer.
METHODS
Normal gastric mucosa,chronic superficial gastritis, atrophic gastritis without intestinal metaplasia, atrophic gastritis with intestinal metaplasia, moderate and severe dysplasia, early and advanced gastric cancer, 60 cases each group, were selected for PTEN protein expression by SP immunohistochemistry.
RESULTS
The expression of PTEN encoding product in normal gastric mucosa was 100%. For chronic superficial gastritis, atrophic gastritis without intestinal metaplasia, atrophic gastritis with intestinal metaplasia, moderate dysplasia, severe dysplasia, the PTEN protein expression rate was 98.3%, 91.6%, 78.3%, 75%, 63.3%,respectively. The expression of PTEN protein in early stage and advanced gastric cancer was 61.7% and 43.3% respectively. Among the 120 cases of gastric cancer, 76 cases were intestinal type gastric cancer, the PTEN protein expression was 60.5%, 44 cases were diffuse gastric cancer , the PTEN protein expression was 38.6%.
CONCLUSION
The expression of PTEN protein is downregulated in the process of gastric cancer, PTEN protein can be used as a maker to evaluate the biological behaviours of gastric cancer.
Key Words: N/A
0 引言
PTEN/MMAC1/TEP1 (phosphatase and tensin homologue deleted on chromosome ten/mutated in multiple advanced cancers/TGF-β-regulated and epithelial cell-enriched phosphatase)是 一个新的肿瘤抑制基因[1,2]. PTEN基因异常在肿瘤的发生发展过程中起重要作用[3-5], 与胃癌的浸润和转移有关[6,7], 我们研究PTEN编码产物与胃癌癌前期病变的关系如下.
1 材料和方法
1.1 材料
2001-2002年胃镜中心活检, 正常胃黏膜, 慢性萎缩性胃炎不伴肠化, 慢性萎缩性胃炎伴肠化, 中度不典型增生, 重度不典型增生病例各60例, 年龄29-74 (平均55岁); 肿瘤科术后大体标本, 早期胃癌及进展期胃癌各60例, 术前未化放疗, 年龄36-68 (平均54岁). 经HE染色, 病理医生诊断. PTEN mAb (浓缩液)和S-P试剂盒购自福州迈新公司.
1.2 方法
标本经甲醛固定, 石蜡包埋, 5 μm连续切片用于S-P免疫组织化学染色. PTEN染色定位在细胞质内. 由两位观察者随机选择5个有代表性的视野计数100个细胞来确定PTEN的染色强度. PTEN在组织中的表达强度分级如下: 阳性细胞小于或等于5%为阴性(-); 5-25%为弱阳性(+); 25-50%为阳性(++); 大于50%为强阳性(+++).
统计学处理 采用x2检验比较各组间比率的差异, 应用Spearman分析等级资料. P <0.05为差异具有显著意义, 所有数据均利用SPSS10.0统计学软件处理.
2 结果
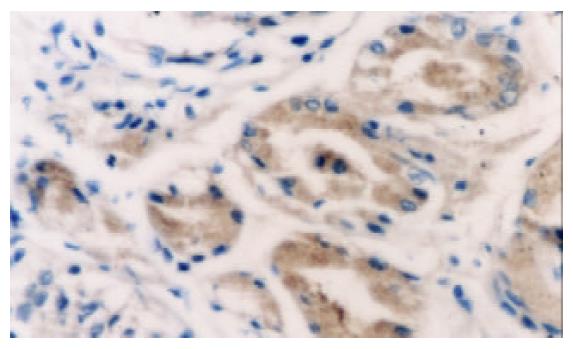
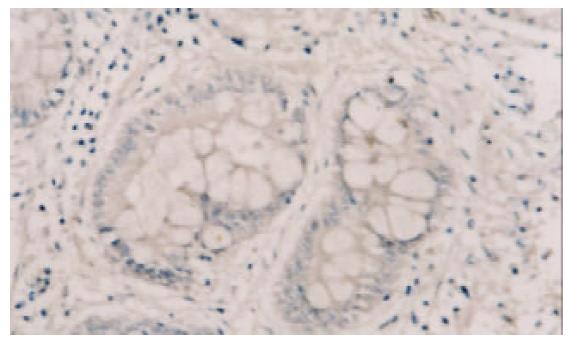
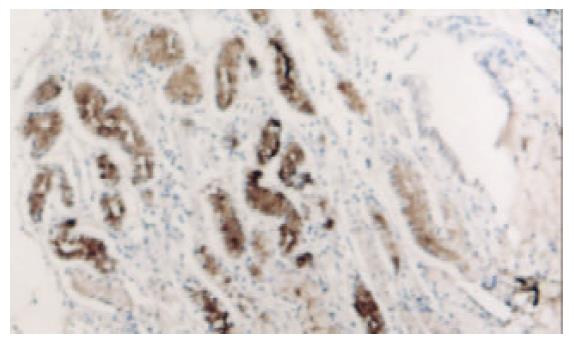
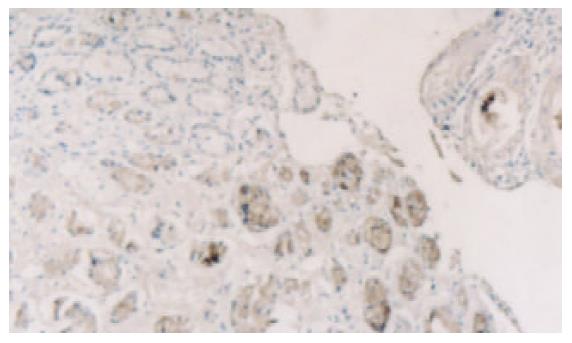
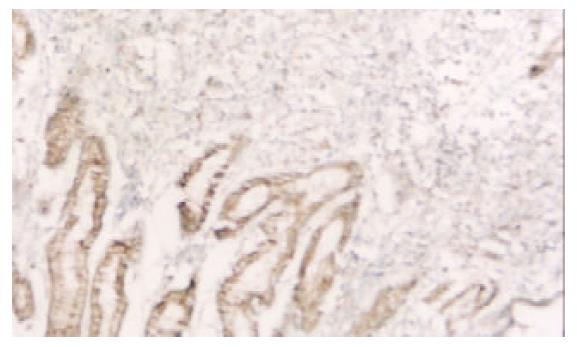
PTEN蛋白在正常胃黏膜、慢性浅表性胃炎、萎缩性胃炎不伴肠化、萎缩性胃炎伴肠化、中度异型增生、重度异型增生、早期胃癌和进展期胃癌的阳性表达率分别为100.0% (60/60), 98.3% (59/60), 91.6% (55/60), 78.3% (47/60), 75.0% (45/60), 63.3% (38/60), 61.7% (37/60), 43.3% (26/60). 所检测的120例胃癌中, 肠型胃癌76例, PTEN表达率为60.5%, 弥漫型胃癌44例, PTEN表达率为38.6%. (图1-6).
图5 早期胃癌PTEN表达降低(左下阳性表达处为正常黏膜)×20.
3 讨论
PTEN在各种癌中总的突变率为5-40%, 其间的差异可能由于组织学类型和临床分期的不同[8-16], 目前认为在具有遗传倾向的结直肠肿瘤如青少年多发性息肉病中发病率较高, 而在散发性结直肠癌中发病率较低[17-19]. 胃癌是常见的消化道肿瘤, 在胃癌的发生中, 从正常胃黏膜上皮转化成癌是一个多步骤的过程, 他是由于多种基因异常在多年的阶段中积累的结果, 其中涉及到多种癌基因, 抑癌基因, 端粒及端粒酶, 细胞黏附因子及DNA错配修复基因的异常和积累[20-34], 本结果表明, 在从慢性胃炎-萎缩性胃炎-肠上皮化生-异型性增生-癌变的过程中, PTEN的阳性表达率逐渐降低, 缺失率逐渐升高. PTEN蛋白在正常胃黏膜中为100%表达, 在慢性浅表性胃炎中的表达率为98.3%, 与正常胃黏膜相比, 无显著差异(x2=0.000, P =1.000). 在无肠化的萎缩性胃炎中的表达率为91.6%, 而在伴有肠化的萎缩性胃炎中表达率为78.3%, 二者之间差异显著(x2=4.183, P =0.041), 而无肠化的萎缩性胃炎与慢性浅表性胃炎相比, 二者间无显著差异(x2=2.807, P =0.094), 说明抑癌基因PTEN对于增生性萎缩性胃炎向恶性转化过程中起重要作用. 不典型增生组PTEN缺失率较萎缩性胃炎伴肠化高, 且有显著差异(x2=4.076, P =0.042), 但对于中度、重度不典型增生, PTEN缺失频率之间无显著差异(x2=1.915, P =0.166), 但Spearman分析结果显示表达强度有差异, 说明随着增生程度的增加, 抑癌基因的缺失也越来越明显. 在从不典型增生向癌的发生过程中, PTEN呈持续性缺失, PTEN在弥漫性胃癌的缺失率较肠型胃癌高, 其间有显著差异(x2=5.355, P =0.021), 说明随着肿瘤恶性程度的增加, PTEN的缺失率增高.
总之, 我们认为抑癌基因PTEN蛋白在胃癌的发生过程中进行性下调, 其缺失在癌前期病变萎缩性胃炎伴肠化阶段就开始出现, 一直持续到进展期胃癌, 并且随着恶性程度的增加, PTEN蛋白缺失率增高, 其机制可能与抑制细胞生长、迁移、铺展和局部黏附, 促进血管形成等有关, 但对于PTEN蛋白表达为原生型还是野生型?这种缺失发生于转录前还是转录后, 还需要通过分子生物学的研究来进一步明确.