修回日期: 2003-03-20
接受日期: 2003-03-28
在线出版日期: 2003-08-15
探讨丁酸钠、穿琥宁体外联合应用对结肠癌细胞的影响.
应用生长曲线、MTT、流式细胞仪、电镜研究丁酸钠、穿琥宁对HCT-8的增生抑制及诱导凋亡作用.
丁酸钠对HCT-8细胞有明显抑制作用, 48 h后各组抑制率分别为15.7%, 20.3%, 33.3% (P<0.01), 呈现浓度、时间依赖性. 电镜下观察可见成熟分化改变及凋亡细胞, 流式细胞仪可检测到亚G1峰, 细胞周期分析发现其主要阻滞在G1期. 穿琥宁与其联合应用, 混合组1在24 h, 48 h凋亡率为23.5%, 48.6%, 混合组2在2 4h, 48 h凋亡率为30.8%, 54.2% (P<0.01).
丁酸钠抑制HCT-8细胞增生, 并诱导其成熟分化、凋亡.穿琥宁与其合用使凋亡率增加.
引文著录: 布立民, 纪欣, 韩英, 陈刚, 王志红, 孙淑红. 丁酸钠联合穿琥宁对人大肠癌细胞 HCT-8 增生的影响. 世界华人消化杂志 2003; 11(8): 1193-1196
Revised: March 20, 2003
Accepted: March 28, 2003
Published online: August 15, 2003
To study the effect of sodium butyrate in combination with chuanhuning on HCT-8 cell line proliferation.
Inhibition of HCT-8 cell line by sodium butyric acid in combination with chuanhuning was detected by MTT assay and growth curve, and apoptosis was determined by morphological assay and flow cytometry (FCM). Apoptotic cells were observed electro- microscopically.
Sodium butyric acid showed inhibitory effect on the proliferation of HCT-8 cell line in dose-dependent and time-dependent manner. The inhibitory rates were 15.7%, 20.3%, and 33.3% (P<0.01) in different groups. Differentiation and apoptosis were observed under electronic microscope. Sub-G1 peak was detected by FCM. Cell cycle was blocked in S phase. The apoptotic rate of combined group 1 were 23.5%, 48.6% at 24 h, and 48 h, and the apoptotic rate of combined group 2 were 30.8%, 54.2% at 24 h, and 48 h (P<0.01).
Sodium butyric acid can induce apoptosis and differentiation of HCT-8 cells of human colorectal carcinoma, and inhibit proliferation of HCT-8 cells. Apoptotic rate was significantly increased when sodium butyric acid was combined with Chuanhuning.
- Citation: Bu LM, Ji X, Han Y, Chen G, Wang ZH, Sun SH. Effect of sodium butyrate combined with chuanhuning on HCT-8 cell line proliferation. Shijie Huaren Xiaohua Zazhi 2003; 11(8): 1193-1196
- URL: https://www.wjgnet.com/1009-3079/full/v11/i8/1193.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i8.1193
大肠癌较常见, 随着人民饮食结构的改变, 大肠癌的发病率与死亡率逐年上升严重威胁人们的身心健康, 平均5 a生存率低于40%[1,2]. 积极探讨大肠癌综合治疗的新手段有重要的临床和社会经济意义. 晚期转移性或不可切除的结肠癌患者的化疗仍以5-FU为主, 但总有效率仍不满意. 研究表明, 多种肿瘤的发生与细胞凋亡障碍有关[3,4], 诱导肿瘤细胞凋亡可能成为肿瘤治疗中有前景的手段之一.丁酸钠作用于大肠癌细胞株, 可抑制其增生, 阻滞细胞周期, 并诱导细胞凋亡[5-11], 而穿琥宁与其联合应用, 使抑制作用明显增强, 凋亡率增加, 这为大肠癌的治疗及预防提供了新途径.
穿琥宁由哈尔滨制药三厂生产, 有效成分为脱水穿心莲内酯琥珀酸半酯单钾盐, 200 mg溶于PBS溶液5 mL中, 4 °C室温避光保存. 丁酸钠 (n-butyric acid sodium)购自Sigma公司, 250 mg溶于PBS溶液20 mL中, 4 °C室温保存, 保存液浓度为0.11 mol/L, 噻唑兰(MTT)、碘化丙啶(PI) Sigma公司产品. HCT-8为结肠腺癌细胞, 由北京药物研究所提供, 贴壁培养于100 mL/L胎牛血清和青、链霉素的RPMI1640的培养液中, 置37 °C, 50 mL/L CO2饱和湿度的无菌培养箱中培养.
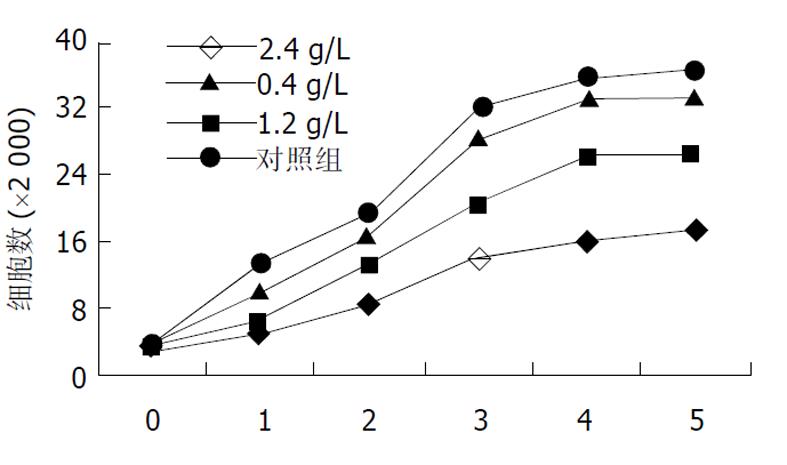
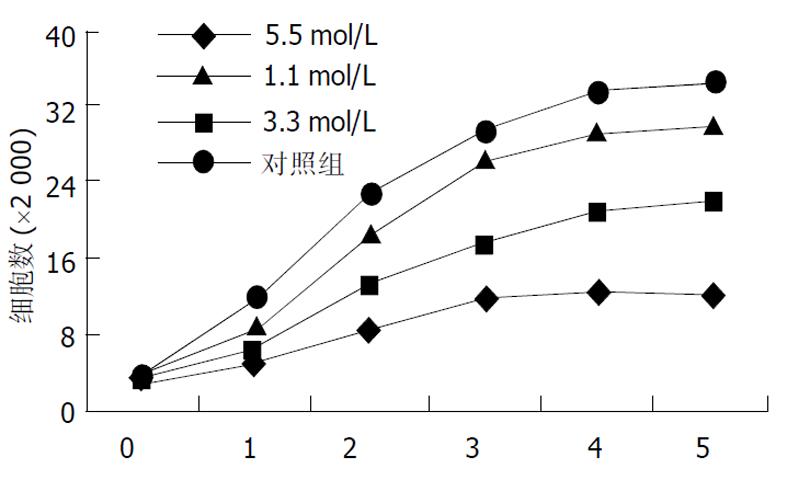
将对数期生长细胞用胰酶消化成单个分散细胞, 分种到每孔含3 mL培养液的6孔板中, 培养24 h后加入穿琥宁溶液和丁酸钠溶液, 各组的终浓度分别为: 穿琥宁0.4 g/L, 1.2 g/L, 2.4 g/L, 丁酸钠1.1, 3.3, 5.5 mol/L, 连续培养5 d, 每天每组取3孔进行细胞计数, 每组取平均值绘制生长曲线. 根据生长曲线观察结果, 分为穿琥宁1组(0.4 g/L)、穿琥宁2组(1.2 g/L)、穿琥宁3组(2.4g/L)、丁酸钠1组(1.1mol/L)、丁酸钠2组(3.3mol/L)、丁酸钠3组(5.5mol/L)及混合组1(穿琥宁1.2 g/L+丁酸钠3.3 mol/L)、混合组2(穿琥宁1.2 g/L+丁酸钠5.5 mol/L)进行细胞毒性试验及流式细胞仪检测.
1.2.1 细胞毒性试验 取对数生长期细胞调整至5×108/L接种到96孔板, 使每孔含培养液100 μL, 细胞数5×104/孔, 培养24 h后, 分别加入上述浓度的丁酸钠及穿琥宁, 作用48 h后每孔加MTT10 μL, 继续培养4 h后弃上清液, 每孔加二甲基亚砜100 μL, 振荡10 min, 使各孔结晶充分溶解, 30 min后采用波长490 nm在酶标仪上测定各孔A值, 每浓度组取8个复孔, 取平均值按下式计算抑制率: 抑制率(%)=(对照组A值-实验组A值)/对照组A值×100%.
1.2.2 流式细胞仪分析 取对数期生长细胞以5×108/L, 接种于50 mL培养瓶中, 加等量PBS溶液为阳性对照组, 适应性培养24 h后, 各实验组分别加入相应浓度的药物, 在加药后24、48 h终止培养, 胰酶消化成单细胞悬液, 进行碘化丙啶(PI)染色, 流式细胞仪检测, 用MμLticycle软件进行 DNA含量和细胞周期分析.
1.2.3 透射电镜观察 取正常对照组、丁酸钠3.3 mmol组、穿琥宁1.2 mg组和混合组1在加药后24 h用少量胰酶消化脱壁, 25 g/L戊二醛固定后, 常规脱水染色, 制成超薄切片后进行电镜观察.
统计学处理 采用t检验方法, P<0.01, 数据分析软件为STATA软件.
低浓度穿琥宁 (0.4 g/L)对细胞生长无明显抑制作用, 1.2 g/L穿琥宁对细胞生长有轻度抑制作用, 2.4 g/L浓度有一定抑制, 但细胞仍可生长(图1).丁酸钠各浓度均有抑制作用, 1.1 mol/L组轻度抑制, 3.3 mol/L中度抑制, 5.5mol/L有明显抑制作用(图2).
根据各组PI值进行抑制率的比较, 对照组为3%, 穿琥宁0.6g/L组为7%, 穿琥宁1.2g/L组为13%, 穿琥宁2.4 g/L组为24%, 丁酸钠1.1 mol/L组为15.7%, 丁酸钠3.3 mol/L组为20.3%, 丁酸钠5.5 mol/L组为33.3%, 混合组1为40%, 混合组2为63.1%. 穿琥宁各组对HCT-8细胞的毒性作用并不明显, 丁酸钠各组随着剂量增加, 抑制程度更明显, 而混合组抑制明显, 超过各组的叠加作用.
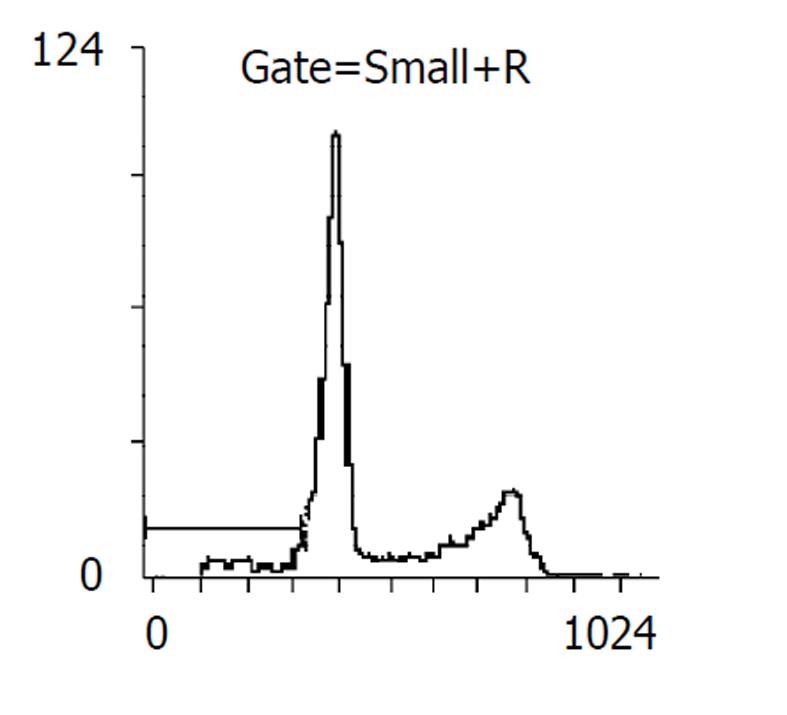
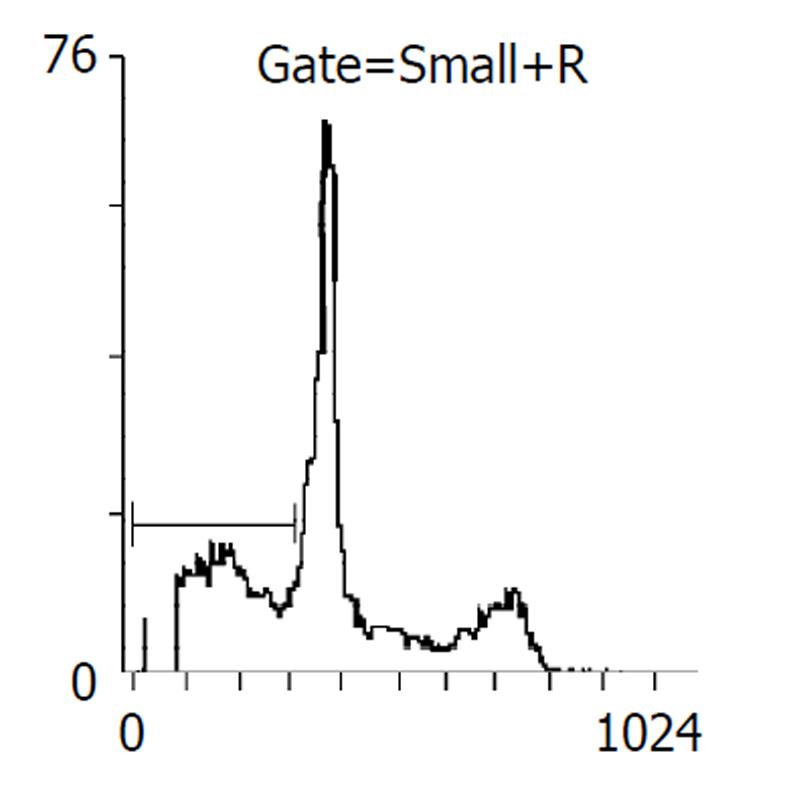
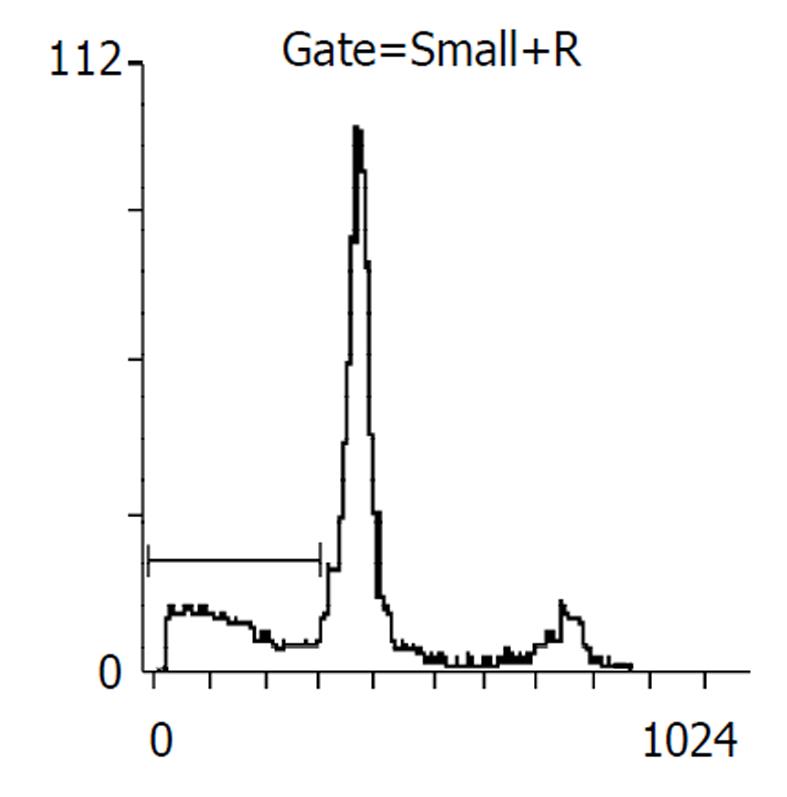
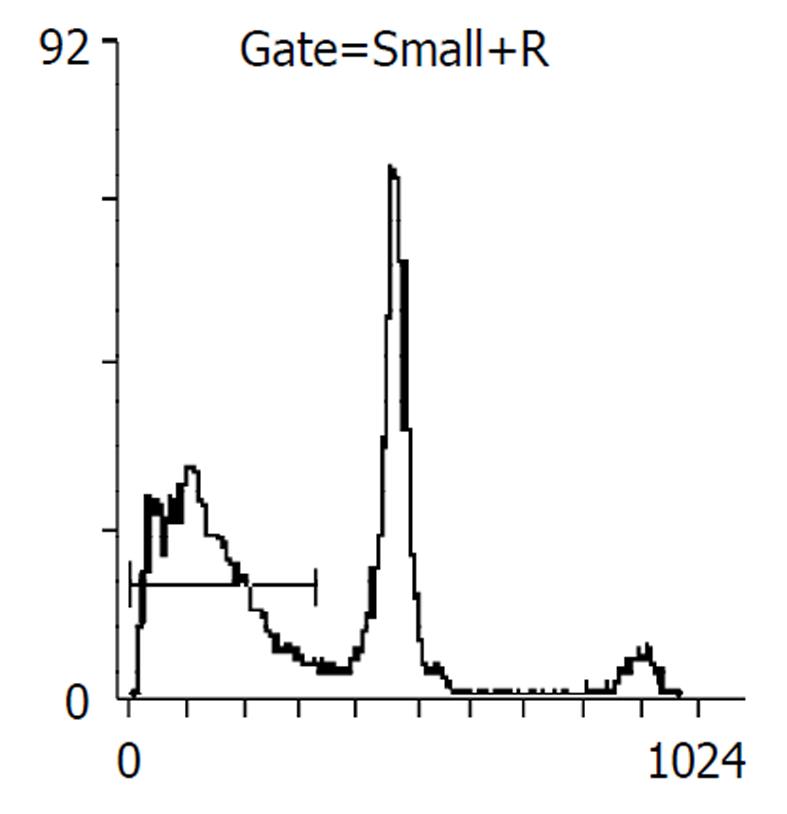
药物作用后24, 48 h分别收集细胞, 比较各处理组凋亡率, 阴性对照分别为2%, 5%, 穿琥宁1.2 g/L组为3%, 5%, 穿琥宁2.4 g/L组为4%, 9.2%, 丁酸钠3.3mol/L组为13.7%, 17%, 丁酸钠5.5 mol/L组为14.1%, 23%, 混合组1为23.5%, 48.6%, 混合组2为30.8%, 54.2%. 且作用48 h后, 各混合组均出现明显的亚G1峰, (见图3, 图4, 图5, 图6)细胞周期分析显示与对照组比较, 穿琥宁对细胞周期无明显影响, 而丁酸钠及混合组细胞均可见G1期细胞增加, 由54%增加到72%, S期、G2细胞数量下降, 由46%下降到28%说明丁酸钠可能影响细胞S期合成, 使细胞阻滞在G1期.
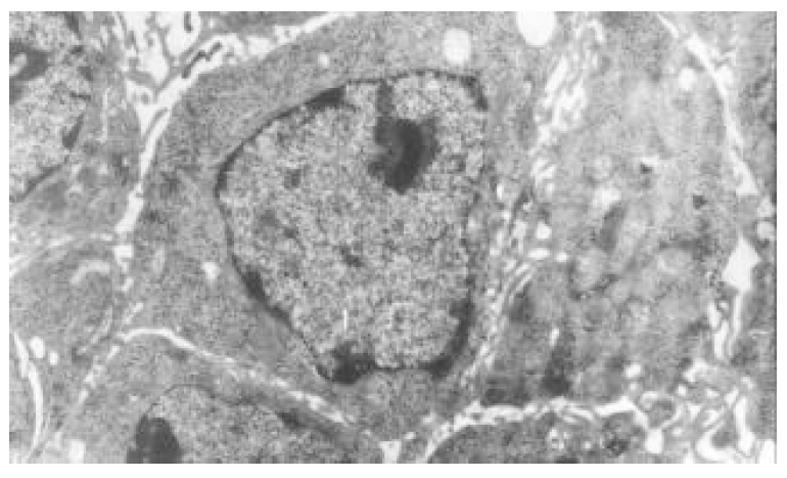
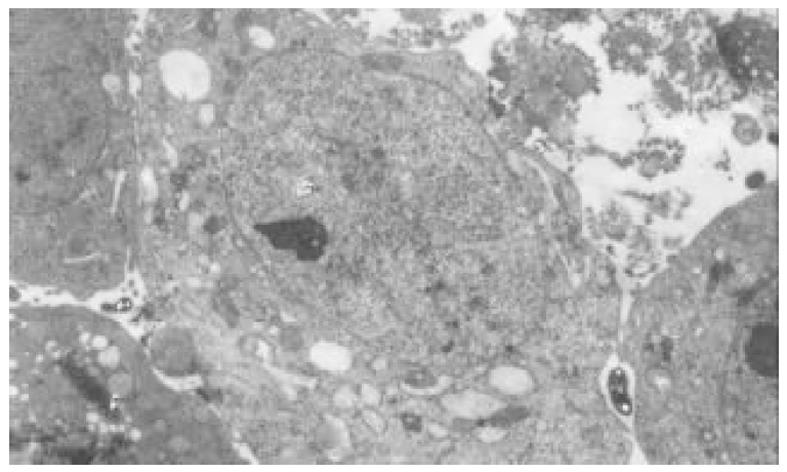
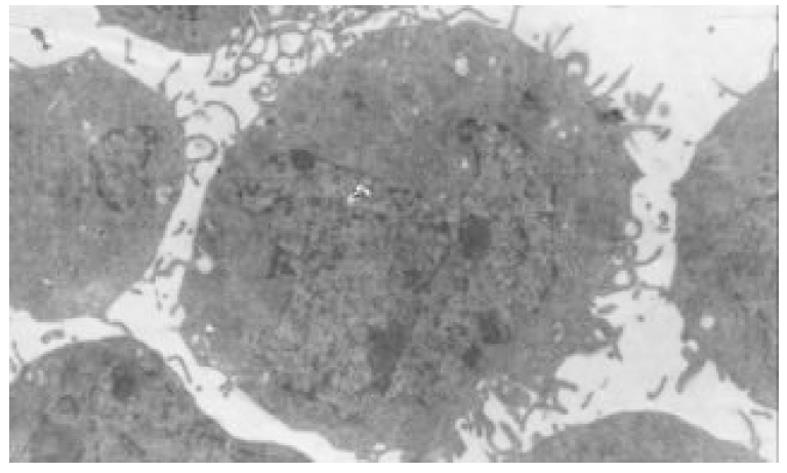
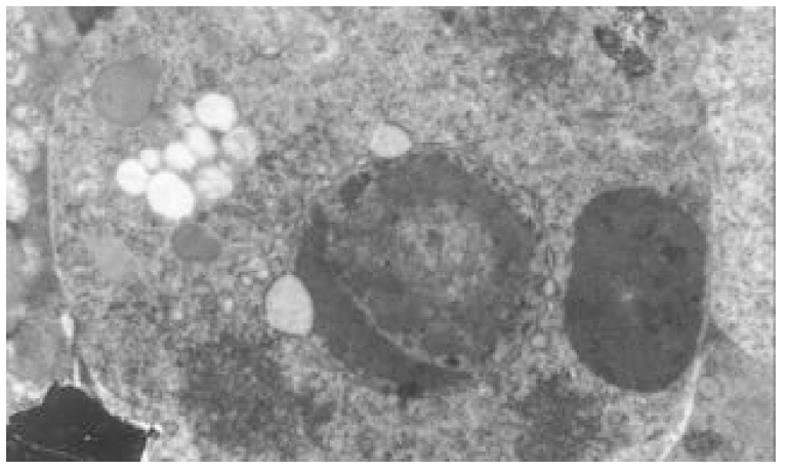
正常HCT-8细胞核大, 外形不规则, 细胞质中细胞器稀少, 常染色体丰富, 异染色体散在分布(图7); 丁酸钠诱导后, 细胞核较规则, 核浆比例减少, 常染色体向核边浓缩, 异染色质稀少, 多数细胞内质网、高尔基体数量、密度明显增多, 部分细胞细胞质呈大量空泡样改变(图8). 穿琥宁作用后, 细胞外形圆形改变, 胞膜外微绒毛明显增多, 核浆比例减少(图9). 混合组作用后, 可见大量细胞呈凋亡形态, 细胞核皱缩, 染色质浓缩于核边, 呈半月形、环形, 部分细胞细胞质内可见凋亡小体(图10).
大肠癌的发生表现为多基因、多步骤的协同累积作用. 丁酸盐对大肠癌的作用机制是目前研究热点[12-21]. 丁酸钠可抑制大肠癌细胞生长, 诱导癌细胞成熟分化[22-24]. 丁酸钠还有诱导多种癌细胞发生凋亡的作用并参与多种基因的表达[26-28]. 本实验证实, 生理浓度下单独应用丁酸钠不仅可以抑制大肠癌HCT-8细胞增生, 而且在一定程度上诱发细胞凋亡, 电镜下可见到细胞核固缩, 染色质浓缩等典型凋亡表现, 未凋亡细胞也有胞核较规则, 核质比例缩小, 异染色质减少, 多数细胞内质网、高乐基体数量及密度明显增加等成熟分化的表现. G1/G0期是细胞群比例增加, G1期延长, G1期向S期过渡是细胞开始分化早期效应, 累积于G1期的细胞是一些分化或将要分化的细胞群体[29,30]. 丁酸钠抑制大肠癌细胞生长, 诱发凋亡的机制尚未清楚. 已知丁酸钠可导致细胞核内多种变化, 包括组蛋白的超大型乙酰化、DNA的甲基化及非组蛋白HMG (high mobility group)的修饰. 其中以组蛋白的超乙酰基化最为重要, 与基因表达和抑制细胞生长有关.丁酸作为乙酰化酶的抑制剂, 使组蛋白超乙酰化后, 能够诱导抑制多种基因的转录, 促进细胞分化, 诱导细胞周期阻滞和细胞凋亡.丁酸钠诱导细胞凋亡的能力可能是饮食中摄入纤维能预防大肠癌发生的机制之一.
穿琥宁为脱水穿心莲内酯琥珀酸半酯单钾盐的干粉制剂, 对W256细胞有一定的抑制作用, 其精氨酸复盐能抑制大鼠乳腺癌SHZ-88细胞株生长, 随浓度加大, 抑制作用加强并能抑制其DNA合成, 硒化穿心莲内酯对小鼠肉瘤180的抑制率为74-80%, 此外穿心莲内酯具有较强的促急性骨髓白血病M1细胞分裂活性.穿心莲注射治疗对绒毛上皮癌及恶性葡萄胎有一定疗效, 有报道穿琥宁静滴能使HCG迅速下降, 于治疗后期辅以抗癌药疗效更佳.
我们曾做过中药提取物与化疗药品联合应用对大肠癌细胞株的相关研究[31], 作者认为单独应用丁酸钠对人大肠癌HCT-8细胞有一定抑制作用, 且随时间、剂量的增加而增强, 透射电镜及流式细胞式均可检测到细胞凋亡, 与相关文献报道相同. 单独应用穿琥宁对HCT-8细胞, 低浓度无明显抑制作用, 高浓度有轻度抑制作用. 单独应用穿琥宁不会导致细胞凋亡. 穿琥宁与丁酸钠合用, 与单独应用丁酸钠相比, 对HCT-8细胞有显著抑制作用, 与单独应用丁酸钠对照, 细胞凋亡率明显增高. 穿琥宁对丁酸钠增敏作用的机制尚未清楚. 明确丁酸钠和穿琥宁联合对大肠癌HCT-8细胞的作用机制, 将为大肠癌的预防与治疗提供一种新途径.
| 2. | Fernandez E, Porta M, Malats N, Belloc J, Gallen M. Symptom-to-diagnosis interval and survival in cancers of the digestive tract. Dig Dis Sci. 2002;47:2434-2440. [PubMed] [DOI] |
| 3. | Strul H, Arber N. Non-steroidal anti-inflammatory drugs and selective apoptotic anti-neoplastic drugs in the prevention of colorectal cancer: the role of super aspirins. Isr Med Assoc J. 2000;2:695-702. [PubMed] |
| 5. | Avivi-Green C, Polak-Charcon S, Madar Z, Schwart ZB. Different molecular events account for butyrate-induced apoptosis in two human colon cancer cell lines. J Nutr. 2002;132:1812-1818. [DOI] |
| 6. | Wang Q, Li N, Wang X, Kim MM, Evers BM. Augmentation of sodium butyrate-induced apoptosis by phosphatidylinositol 3-kinase inhibition in the KM20 human colon cancer cell line. Clin Cancer Res. 2002;8:1940-1947. [PubMed] |
| 7. | Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. 2002;132:1012-1017. [PubMed] [DOI] |
| 8. | Oliver L, Cordel S, Barbieux I, LeCabellec MT, Meflah K, Gregoire M, Vallette FM. Resistance to apoptosis is increased during metastatic dissemination of colon cancer. Clin Exp Metastasis. 2002;19:175-180. [DOI] |
| 9. | Tan S, Seow TK, Liang RC, Koh S, Lee CP, Chung MC, Hooi SC. Proteome analysis of butyrate-treated human colon cancer cells (HT-29). Int J Cancer. 2002;98:523-531. [PubMed] [DOI] |
| 10. | Giermasz A, Grzela T, Nowis D, Makowski M, Czaika A, Stoklosa T, Lasek W, Dabrowsk A, Wlznerowlez M, Macklewlcz A. Butyric acid enhances in vivo expression of hTNF-alpha in transduced melanoma cell line. Anticancer Res. 2001;21:4001-4004. [PubMed] |
| 11. | Salomone B, Ponti R, Gasco MR, Ugazio E, Quaglino P, Osella-Abate S, Bernengo MG. In vitro effects of cholesteryl butyrate solid lipid nanospheres as a butyric acid pro-drug on melanoma cells: evaluation of antiproliferative activity and apoptosis induction. Clin Exp Metastasis. 2000;18:663-673. [DOI] |
| 12. | Sasahara Y, Mutoh M, Takahashi M, Fukuda K, Tonaka N, Sugimura T, Wakaboyashi K. Suppression of promoter-dependent transcriptional activity of inducible nitric oxide synthase by sodium butyrate in colon cancer cells. Cancer Lett. 2002;177:155-161. [DOI] |
| 13. | Leng SL, Leeding KS, Gibson PR, Bach LA. Insulin-like growth factor-II renders LIM 2405 human colon cancer cells resistant to butyrate-induced apoptosis: a potential mechanism for colon cancercell survival in vivo. Carcinogenesis. 2001;22:1625-1631. [DOI] |
| 14. | Hernandez A, Thomas R, Smith F, Sandberg J, Kim S, Chung DH, Evers BM. Butyrate sensitizes human colon cancer cells to TRAIL-mediated apoptosis. Surgery. 2001;130:265-272. [PubMed] [DOI] |
| 15. | Iacomino G, Tecce MF, Grimaldi C, Tosto M, Russo GL. Transcriptional response of a human colon adenocarcinoma cell line to sodium butyrate. Biochem Biophys Res Commun. 2001;285:1280-1289. [PubMed] [DOI] |
| 16. | Rosato RR, Wang Z, Gopalkrishnan RV, Fisher PB, Grant S. Evidence of a functional role for the cyclin-dependent kinase-inhibitor p21WAF1/CIP1/MDA6 in promoting differentiation and preventing mitochondrial dysfunction and apoptosis induced by sodium butyrate in human myelomonocytic leukemia cells (U937). Int J Oncol. 2001;19:181-191. [DOI] |
| 17. | Wachtershauser A, Akoglu B, Stein J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis. 2001;22:1061-1067. [DOI] |
| 18. | Bidon N, Brichory F, Thomas D, Cavalier A, Caulet-Maugendre S, Bourguet P, Dazord L. Sodium butyrate induces growth inhibition and modulates galectin-8 expression inhuman lung carcinoma cells. Anticancer Res. 2001;21:1049-1055. [PubMed] |
| 19. | Miyake H, Hara S, Arakawa S, Kamidono S, Hara I. Overexpression of Bcl-2 regulates sodium butyrate- and/or docetaxel-induced apoptosis in human bladder cancer cells both in vitro and in vivo. Int J Cancer. 2001;93:26-32. [PubMed] [DOI] |
| 20. | Rabizadeh E, Bairey O, Aviram A, Ben-Dror I, Shaklai M, Zimra Y. Doxorubicin and a butyric acid derivative effectively reduce levels of BCL-2 protein in the cells of chronic lymphocytic leukemia patient. Eur J Haematol. 2001;66:263-271. [DOI] |
| 21. | Bras-Goncalves RA, Pocard M, Formento JL, Poirson-Bichat F, De Pinieux G. Synergistic efficacy of 3n-butyrate and 5-fluorouracil in human colorectal cancer xenografts via modulation of DNA synthesis. Gastroenterology. 2001;120:874-888. [DOI] |
| 22. | Wachtershauser A, Stein J. Butyrate-induced differentiation of Caco-2 cells occurs independently from p27. Biochem Biophys Res Commun. 2001;281:295-299. [PubMed] [DOI] |
| 23. | Wu JT, Archer SY, Hinnebusch B, Meng S, Hodin RA. Transient vs. prolonged histone hyperacetylation: effects on colon cancer cell growth, differentiation, and apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G482-490. [PubMed] [DOI] |
| 24. | Milovic V, Teller IC, Turchanowa L, Caspary WF, Stein J. Effect of structural analogues of propionate and butyrate on colon cancer cell growth. Int J Colorectal Dis. 2000;15:264-270. [DOI] |
| 25. | Karasawa Y, Murakami A, Okisaka S. Apoptosis after butyrate-induced differentiation in retinoblastoma cell line Y-79. Jpn J Ophthalmol. 2000;44:601-609. [DOI] |
| 26. | Hara I, Miyake H, Hara S, Arakawa S, Kamidono S. Sodium butyrate induces apoptosis in human renal cell carcinoma cells and synergistically enhances their sensitivity to anti-Fas-mediated cytotoxicity. Int J Oncol. 2000;17:1213-1218. [DOI] |
| 27. | Tsai LC, Hung MW, Chang GG, Chang TC. Apoptosis induced by the sodium butyrate in human gastric cancer TMK-1 cells. Anticancer Res. 2000;20:2441-2448. [PubMed] |
| 28. | Litvak DA, Hwang KO, Evers BM, Townsend CM Jr. Induction of apoptosis in human gastric cancer by sodium butyrate. Anticancer Res. 2000;20:779-784. [PubMed] |