修回日期: 2001-10-30
接受日期: 2001-11-02
在线出版日期: 2003-08-15
探讨垂体后叶素和特利加压素降低肝硬化门脉高压时对肝组织氧分压的不同影响.
采用胆总管结扎法制作胆汁性肝硬化模型, 40只大鼠随机分成2组: 垂体后叶素组(n1 = 20), 特利加压素组(n2 = 20), 两组分别从门静脉缓慢注入垂体后叶素和特利加压素, 连续观察用药前及用药后5, 10, 15, 20, 25, 30 min的门静脉压及肝组织氧分压.
两组用药后门静脉压均明显降低, 两组间无显著差异(t = 0.39 P>0.05); 垂体后叶素组用药后肝组织氧分压明显下降, 特利加压素组无明显下降, 两组间有显著差异(t = 9.19 P<0.01).
垂体后叶素降低门静脉压时肝组织氧分压明显下降, 而特利加压素在降低门静脉压时肝组织氧分压无明显降低, 是治疗急性食管、胃静脉曲张破裂出血较理想的药物.
引文著录: 祝建波, 邓利群, 王思元. 垂体后叶素和特利加压素降低门胆汁性肝硬化大鼠门静脉高压对肝组织氧分压的影响. 世界华人消化杂志 2003; 11(8): 1172-1174
Revised: October 30, 2001
Accepted: November 2, 2001
Published online: August 15, 2003
To explore the different effects of pituitrin and triglycyl-lysine-vasopressin (tGLVP)on hepatic oxygen partial pressure (PhO2) while reducing the partial hypertension in portal hypertension rats.
The model of biliary cirrhosis was induced by ligating the choledochus. Forty rats were divided into two groups randomly: pp group (n = 20)and tGLVP group(n = 20). PP and tGLVP were slowly infused into the rats via portal vein respectively. The portal vein pressure and the oxygen partial pressure were obtained at the moment before administration and 5, 10, 15, 25, 30 minutes after administration.
Portal venous pressure (PVP) was significantly lowered after infusion in two groups. There was no significant difference between the two groups (t = 0.39, P>0.05). PhO2 was significantly decreased in pp group after infusion (P<0.01), but not in tGLVP group (P<0.05).There was significant difference between the two groups in PhO2 after infusion (t = 9.19, P<0.01).
PP caused a significant decrease both in portal pressure and PhO2. PVP was decreased by tGLVP, but PhO2 was not decreased significantly. tGLVP is considered to be better than PP in treatment of acute variceal hemorrhage.
- Citation: Zhu JB, Deng LQ, Wang SY. Effects of pituitrin and triglycyl-lysine-vasopressin on hepatic oxygen partial pressure in portal hypertensive rats. Shijie Huaren Xiaohua Zazhi 2003; 11(8): 1172-1174
- URL: https://www.wjgnet.com/1009-3079/full/v11/i8/1172.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i8.1172
门静脉高压症(PHT)常见, 其并发症-食管胃静脉曲张出血(EGVB)严重地威胁着患者的生命[1-6]. 垂体后叶素和特利加压素(triglycyl-lysine vasopressin, tGLVP)仍是目前治疗急性EGVB的第一线药物[7], 他们降低门静脉压PVP时对肝脏供血供氧的影响一直是许多研究的热点, 但迄今为止的研究都是通过有效肝血流量(EHBF)来评价他们对肝脏供血供氧的影响[8-14]. 由于肝脏由肝动脉和门静脉双重供血, 而且PHT时肝动脉、肝静脉、门静脉之间均可发生短路, 因此仅用EHBF来评价肝脏供血供氧状况显然不够准确. 氧分压传感针能在体、实时、动态监测体内组织中的氧分压, 已用于实验研究[15-20]. 为了解组织缺血缺氧状态提供了新的手段. 我们以胆汁性肝硬化大鼠模型为对象, 测定了分别静脉注射垂体后叶素和tGLVP前后肝硬化大鼠的PVP和肝组织氧分压(PhO2), 旨在探讨垂体后叶素和tGLVP降低PVP时对肝组织供氧的不同影响, 为临床安全用药提供实验依据.
♂Wistar大鼠40只, 质量(281±11) g, 由华中科技大学同济医学院实验动物学部提供. 垂体后叶素由上海生物化学制药厂生产, tGLVP由瑞典FERRINGAB厂生产. SMUP-PC型生物信号处理系统, 由华中科技大学同济医学院生理教研室提供. 三通道组织氧测定仪由华中科技大学同济医学院生物工程研究室提供. 大鼠实验前禁食24 h, 自由饮水, 30 g/l戍巴比妥钠溶液腹腔注射麻醉(剂量1 ml/kg体重). 常规消毒、铺巾、沿腹白线切开入腹, 暴露并游离胆总管, 在近肝门和近十二指肠处分别结扎胆总管, 并从中剪断胆总管, 内脏复位后, 腹腔注射青霉素20万u, 缝合腹壁切口, 用复合饲料饲养6 wk即建成胆汁性肝硬化模型. 将40只模型大鼠随机分为2组: 垂体后叶素组(n1 = 20)从门静脉缓慢注入垂体后叶素(0.4 u/kg) 1 min内注完; tGLVP 组(n2=20)从门静脉缓慢注入tGLVP (40 ug/kg)1 min内注完.
大鼠实验前禁食24 h, 自由饮水. 30 g/l戍巴比妥钠溶液腹腔注射麻醉(剂量0.5 ml/kg), 常规消毒、铺巾、沿腹白线切开入腹, 分离门静脉主干, 将细塑料管经回结肠静脉插入门静脉主干, 连接SMUP-PC型生物信号处理系统; 用酒精棉球擦洗大鼠尾, 并将大鼠尾尖3 cm部分浸入置有生理盐水的烧杯中, 将氧分压传感针也置入烧杯中调零, 然后, 暴露剑突下肝脏, 将氧分压传感针刺入肝组织中(5 mm), 室内温度保持在25 °C左右, 待术后大鼠生理调节稳定20 min后, 从组织氧测定仪和生物信号处理系统显示屏上读出并记录用药前的PhO2和PVP. 然后从SMVP-PC型生物信号处理系统的三通接头注入VP 0.4 u/kg (VP组)或tGLVP 40 ug/kg (tGLVP组), 从显示屏读出并记录用药后5.10.15.25. 30 min时的PhO2和PVP. 实验结束后取肝组织于40 g/l甲醛缓冲液固定, 常规脱水、包埋、切片、HE染色后用光学显微镜观察.
统计学处理 所有值均用mean±SD表示, 组间比较采用t检验, 规定P<0.05为有显著性差异.
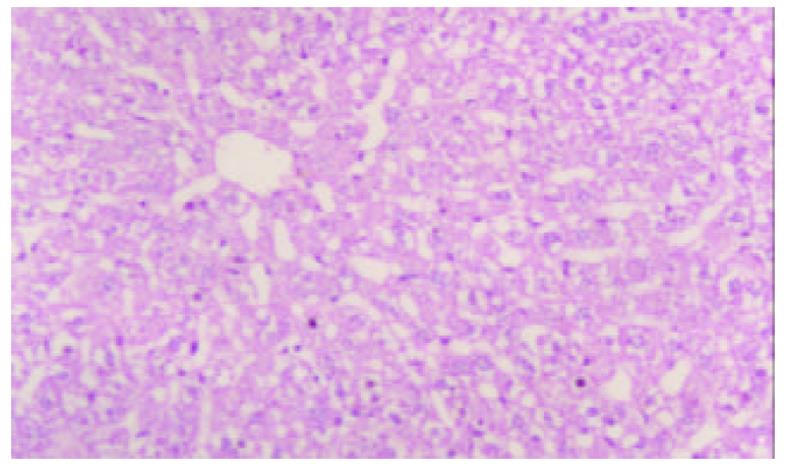
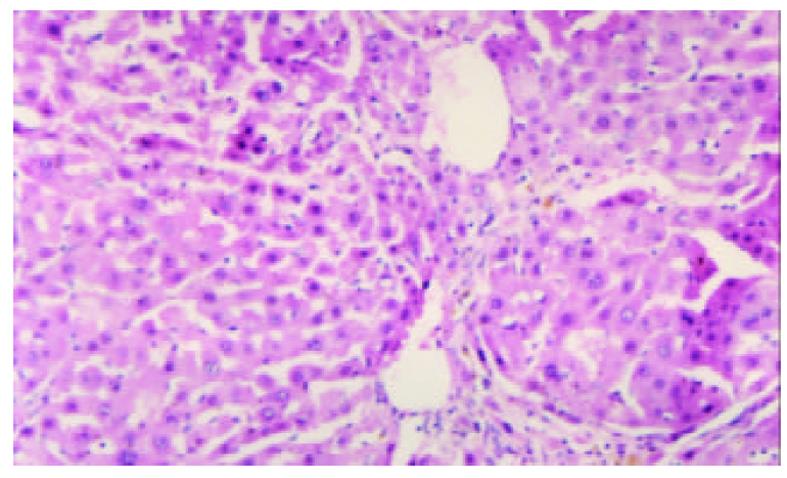
模型制备6 wk后, 模型组所有大鼠均出现显著黄疸、胃肠瘀血、脾肝肿大及腹水. 肝脏表面呈细颗粒状, 被胆汁染成绿褐色. 组织学检查显示胆汁性肝硬化特征性表现, 即光镜下可见汇管区胆管明显扩张, 结缔组织增生显著, 有较多的新生胆管及炎性细胞浸润, 增生的结缔组织向小叶间及小叶内伸延分隔形成假小叶, 小叶内毛细胆管高度扩张, 扩张的胆管破裂形成胆湖 (见图1, 图2).
用药前垂体后叶素组PVP为2.02±0.01 kpa, tGLVP组为2.02±0.07 Kpa. 两组间无明显差异(t = 0.39 P>0.05). 用药后垂体后叶素组各时间段的PVP均较用药前明显下降(P<0.01)用药后 tGLVP组各时段PVP均较用药前明显下降(P<0.01). 两组间用药后各时段的PVP均无明显差异(P>0.05, 表1).
| 分组 | 用药前 | 用药后 (min) | |||||
| 5 | 10 | 15 | 20 | 25 | 30 | ||
| PVP 垂体后叶素 | 2.01±0.07 | 1.71±0.09b | 1.54±0.08b | 1.48±0.07b | 1.490±0.06b | 1.51±0.06b | 1.52±0.06b |
| tGLVP | 2.02±0.10 | 1.73±0.23b | 1.61±0.22b | 1.50±0.06b | 1.48±0.06b | 1.46±0.06b | 1.47±0.06b |
| PhO2 垂体后叶素 | 11.95±0.27 | 10.76±0.46d | 10.34±0.48d | 10.16±0.46d | 10.01±0.49d | 10.02±0.50d | 9.93±0.46d |
| tGLVP | 12.04±0.41 | 11.99±0.37 | 11.95±0.39 | 12.12±0.38 | 11.95±0.42 | 11.97±0.31 | 12.01±0.36 |
用药前垂体后叶素组PhO2为11.95±0.27 kpa, tGLVP组为12.04±0.41 kpa, 两组间无明显差异(t = 0.39 P>0.05), 垂体后叶素组用药后各时段的PhO2均较用药前明显下降(P<0.01), tGLVP组用药后各时段的PhO2较用药前无明显下降(P>0.05), 但两组各时段间的PhO2均有显著差异(t = 9.19 P<0.01, 表1).
垂体后叶素能明显降低PHT患者的PVP, PVBF, EHBF, 心输出量(CO)并引起平均动脉压增高, 但HAF并不增高[8,11,21-26]. 垂体后叶素降低PVP时是否引起肝细胞缺氧, 迄今为止的研究都是通过垂体后叶素对EHBF的影响来间接评价的, 由于肝脏血液供应的特殊性和PHT时肝内微循环发生紊乱. 此种评价方法显然存在着局限性[18,21,23,27]. 我们首次通过PhO2的测定证实了垂体后叶素在降低PHT大鼠的PVP时PhO2也明显下降(P<0.01), 为研究VP降低PVP时对肝脏供血供氧的影响提供了一种新的方法. 有的学者研究认为影响缺血性肝炎通常是由于肝组织低灌注所致, 当EHBF下降时可以引起肝细胞缺氧, 严重缺氧导致肝细胞损害. 垂体后叶素在降低PVP、PVBF的同时明显减少EHBF, PhO2也明显下降, 因而临床上单用垂体后叶素治疗急性EGVB完全有可能诱发或加重肝硬化患者的缺血性肝炎, 应引起临床医师的高度重视.
tGLVP是人工合成的三甘氨酸-赖氨酸-加压素, 是一种无生物活性的加压素, 静脉注射后, 在体内缓慢的转化为有活性的赖氨酸-加压素[11,15,23]. 这种缓慢释放的机制使得tGLVP在一次给药后可维持平滑肌收缩长达成10 h. tGLVP和垂体后叶素对PVP、PVBF、平均动脉压的影响是相似的, 二者之间最重要的差别是当PVP和PVBF下降时, tGLVP能使HAF代偿性的明显增加, 许多研究还表明[14,21]tGLVP不降低PHT的EHBF. 本结果表明, tGLVP在明显降低PVP时PhO2并无明显下降(P>0.05), 这可能与tGLVP降低PVP、PVBF时EHBF并不减少以及HAF代偿性增加有关. 由于tGLVP作用时间长, 无心脏作用, 几乎没有血栓溶解作用, 且降低PVP时对PhO2无明显影响, 因而是优于垂体后叶素治疗急性EGVB的较理想的药物.
| 8. | Bhasin DK, Malhi NJ. Variceal bleeding and portal hypertension: much to learn, much to explore. Endoscopy. 2002;34:119-128. [PubMed] [DOI] |
| 9. | Lin HC, Yang YY, Hou MC, Huang YT, Lee WC, Lee FY, Chang FY, Lee SD. Hemodynamic effects of a combination of octreotide and terlipressin in patients with viral hepatitis related cirrhosis. Scand J Gastroenterol. 2002;37:482-487. [DOI] |
| 10. | Ramirez MC, Martinez-Cuesta MA, D'Ocon P, Noguera MA, Garcia-Zaragoza E, Bosch J, Melin P, Esplugues JV. Comparative effects of the novel vasotocin analogue F-180 vs. vasopressin and terlipressin on systemic and splanchnic isolated vessels from portal hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:199-204. [PubMed] [DOI] |
| 11. | Ramirez MC, Martinez-Cuesta MA, D'Ocon P, Noguera MA, Garcia-Zaragoza E, Bosch J, Melin P, Esplugues JV. New method of cardiac output measurement using ultrasound velocity dilution in rats. J Appl Physiol. 2001;91:1274-1282. [PubMed] [DOI] |
| 12. | Garcia N Jr, Sanyal AJ. Portal hypertension. Clin Liver Dis. 2001;5:509-540. [DOI] |
| 13. | Vlavianos P, Westaby D. Management of acute variceal haemorrhage. Eur J Gastroenterol Hepatol. 2001;13:335-342. [PubMed] [DOI] |
| 14. | Lee WC, Lin HC, Yang YY, Hou MC, Lee FY, Chang FY, Lee SD. Hemodynamic effects of a combination of prazosin and terlipressin in patients with viral cirrhosis. Am J Gastroenterol. 2001;96:1210121-1210126. [PubMed] [DOI] |
| 15. | Hansen EF, Bendtsen F, Brinch K, Moller S, Henriksen JH, Becker U. Endoscopic Doppler ultrasound for measurement of azygos blood flow. Validation against thermodilution and assessment of pharmacological effects of terlipressin in portal hypertension. Scand J Gastroenterol. 2001;36:318-325. [DOI] |
| 16. | Huang HC, Chu CJ, Lee FY, Chang FY, Wang SS, Lin HC, Hou MC, Chan CC, Wu SL, Chen CT. Chronic inhibition of nitric oxide ameliorates splanchnic hyposensitivity to glypressin in a hemorrhage-transfused rat model of portal hypertension. Scand J Gastroenterol. 2000;35:1308-1313. [DOI] |
| 17. | Lee FY, Chu CJ, Wang SS, Chang FY, Lin HC, Hou MC, Chan CC, Wu SL, Chen CT, Huang HC. Inhibition of prostacyclin by indomethacin ameliorates the splanchnic hyposensitivity to glypressin in haemorrhage-transfused common bile duct-ligated rats. Eur J Clin Invest. 2001;31:145-153. [DOI] |
| 18. | Wang SS, Chu CJ, Lee FY, Wu SL, Lin HC, Chan CC, Chang FY, Lee SD. Effects of prostacyclin inhibition on splanchnic hyposensitivity to glypressin in a hemorrhage-transfused rat model of portal hypertension. Scand J Gastroenterol. 2000;35:426-432. [DOI] |
| 19. | Bosch J. The sixth Carlos E. Rubio memorial lecture. prevention and treatment of variceal hemorrhage. PR Health Sci J. 2000;19:57-67. [PubMed] |
| 20. | Romero G, Kravetz D, Argonz J, Bildozola M, Suarez A, Terg R. Terlipressin is more effective in decreasing variceal pressure than portal pressure in cirrhotic patients. J Hepatol. 2000;32:419-425. [DOI] |
| 21. | Arroyo V, Jimenez W. Complications of cirrhosis. II. Renal and circulatory dysfunction. Lights and shadows in an important clinical problem. J Hepatol. 2000;32:157-170. [DOI] |
| 22. | Bosch J, Garcia-Pagan JC. Complications of cirrhosis, I. Portal hypertension. J Hepatol. 2000;32:141-156. [DOI] |
| 23. | Hansen EF, Strandberg C, Hojgaard L, Madsen J, Henriksen JH, Schroeder TV, Becker U, Bendtsen F. Splanchnic haemodynamics after intravenous terlipressin in anaesthetised healthy pigs. J Hepatol. 1999;30:503-510. [DOI] |
| 24. | Oberti F, Veal N, Kaassis M, Pilette C, Rifflet H, Trouve R, Cales P. Hemodynamic effects of terlipressin and octreotide administration alone or in combination in portal hypertensive rats. J Hepatol. 1998;29:103-111. [DOI] |
| 25. | Moller S, Hansen EF, Becker U, Brinch K, Henriksen JH, Bendtsen F. Central and systemic haemodynamic effects of terlipressin in portal hypertensive patients. Liver. 2000;20:51-59. [DOI] |
| 26. | Huang YT, Lin LC, Chern JW, Lin HC, Hong CY. Portal hypotensive effects of combined terlipressin and DL-028, a synthetic alpha 1 adrenoceptor antagonist administration on anesthetized portal hypertensive rats. Liver. 1999;19:129-134. [DOI] |
| 27. | Wolf DC. The management of variceal bleeding: past, present and future. Mt Sinai J Med. 1999;66:1-13. [PubMed] |