修回日期: 2002-09-15
接受日期: 2002-10-18
在线出版日期: 2003-05-15
探讨胃癌中SMAD4/DPC4杂合性丢失(loss of heterozygosity, LOH)与胃癌临床病理的关系.
用多聚酶链反应-单链构相多态性(PCR-SSCP)银染法分析50例原发性胃癌SMAD4/DPC4的杂合性丢失.
D18S46 LOH为36.2% (17/47), D18S474 LOH为39.1%(18/46), DPC4 LOH为59.2% (29/49). SMAD4/DPC4 LOH的发生率随着胃壁浸润深度的加深而增高, 随着TNM分期的增加而增高(P<0.05). 胃癌直径≥5 cm时, SMAD4/DPC4 LOH要明显高于胃癌<5 cm时(P<0.05). SMAD4/DPC4 LOH在Borrmann分型中有显著性差异(P<0.05).
SMAD4/DPC4的杂合性丢失可能在胃癌的发展中起重要作用, 是胃癌发展后期的重要分子事件.
引文著录: 朱亚青, 尹浩然, 朱正纲, 刘炳亚, 张奕, 陈雪华, 于颖彦, 林言箴. 胃癌SMAD4/DPC4杂合性丢失的研究. 世界华人消化杂志 2003; 11(5): 522-525
Revised: September 15, 2002
Accepted: October 18, 2002
Published online: May 15, 2003
To investigate the role of loss of heterozygosity (LOH) of SMAD4/DPC4 and the relationship between the LOH and other clinicopathological factors in gastric carcinoma.
Total 50 cases of gastric carcinoma were monitored with PCR-SSCP and silver staining was performed to detect LOH of SMAD4/DPC4.
The incidence of LOH was found in 36.2% (17/47) of D18S46, 39.1% (18/46) of D18S474 and 59.2% (29/49) of SMAD4/DPC4, respectively. The prevalence of SMAD4/DPC4 LOH increased progressively from intromucosal to advanced deeply invasive cancers (P<0.05). The frequency of SMAD4/DPC4 LOH was significantly higher in TNM Ⅲ and Ⅳ stage than those in TNM Ⅰand Ⅱ stages (P<0.05). SMAD4/DPC4 LOH was statistically significant association with tumor size (P<0.05). And a strong correlation between SMAD4/DPC4 LOH and Borrmann classification was observed (P<0.05).
The SMAD4/DPC4 LOH may promote the progression of gastric carcinoma and is an important molecular event in the development of the tumor at advanced stage.
- Citation: Zhu YQ, Yin HR, Zhu ZG, Liu BY, Zhang Y, Chen XH, Yu YY, Lin YZ. Loss of heterozygosity of SMAD4/DPC4 in gastric carcinoma. Shijie Huaren Xiaohua Zazhi 2003; 11(5): 522-525
- URL: https://www.wjgnet.com/1009-3079/full/v11/i5/522.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i5.522
胃癌是人类最常见的恶性肿瘤之一, 18号染色体长臂(18q)上遗传物质的丢失在许多肿瘤中是一种常见现象[1-8], 特别在胰腺癌和结肠癌中[9-12], 而其在胃癌中的作用还不明确. 我们分析了胃癌标本中位于18q21位点上的SMAD4/DPC4抑癌基因的杂合性丢失, 并探讨其与胃癌临床病理学之间的关系.
瑞金医院2001-2002年手术切除胃癌标本50例, 男29例, 女21例, 年龄59±14岁; 术前未经任何化放疗(表1). 肿瘤根据UICC规定(Sobin L, Wittekind C. TNM classification of malignant tumors. 5th Ed. 1997: 59-62)分型. 新鲜的肿瘤和正常胃组织手术切除后立即用液氮速冻, 并保存在-80 ℃直至DNA抽提.
| 临床指标 | n | 信息个体数 | LOH(%) |
| 男 | 29 | 28 | 17(60.7) |
| 女 | 21 | 21 | 12(57.1) |
| ≤40岁 | 4 | 4 | 2(50.0) |
| >40岁 | 46 | 45 | 27(60.0) |
| 贲门 | 4 | 4 | 2(50) |
| 胃体 | 18 | 18 | 10(55.6) |
| 幽门 | 28 | 27 | 17(63.0) |
| 肿瘤<5 cm | 31 | 31 | 15(48.4)a |
| ≥5 cm | 19 | 18 | 14(77.8) |
| 高分化 | 1 | 1 | 1(100) |
| 中分化 | 19 | 19 | 10(52.6) |
| 低分化 | 30 | 29 | 18(62.1) |
| Laure分型肠型 | 19 | 19 | 10(52.6) |
| 浸润型 | 26 | 25 | 14(56.0) |
| 混合型 | 5 | 5 | 5(100.0) |
| Borrmann分型隆起型 | 3 | 3 | 1(33.3)b |
| 溃疡型 | 34 | 33 | 19(57.6) |
| 弥漫型 | 8 | 8 | 8(100.0) |
| 浸润T1 | 5 | 5 | 1(20.0)c |
| T2 | 8 | 8 | 3(37.5) |
| T3 | 32 | 31 | 20(64.5) |
| T4 | 5 | 5 | 5(100.0) |
| 淋巴结转移 | 24 | 24 | 12(50.0) |
| 无转移 | 26 | 25 | 17(68.0) |
| TNM分期Ⅰ+Ⅱ | 25 | 25 | 11(44.0)d |
| Ⅲ+Ⅳ | 25 | 24 | 18(75.0) |
| 早期胃癌 | 5 | 5 | 1(20.0) |
| 进展期胃癌 | 45 | 44 | 28(63.6) |
1.2.1 方法 所有癌组织均经冰冻切片, HE染色. 连续切10 μm厚的冰冻切片10-20张, 对癌细胞比例在70%以下的标本行显微切割. 显微切割参照Lo et al[13]的方法, 稍作修改. 参照相对应的HE染色切片, 在40倍显微镜下用5号无菌注射器将肿瘤细胞与正常组织切割开来, 将其转入1.5 mL的eppendorf管. 用组织裂解液和蛋白酶K消化, 56 ℃孵育过夜. 标准的酚/氯仿/异戊醇法抽提基因组DNA. 用SMAD4/DPC4侧翼的两个紧密连锁的微卫星位点D18S46和D18S474来分析LOH, 其引物序列见表2(参见http://www.ncbi.nlm.nih.gov/genemap). 引物由上海博亚生物技术有限公司合成. 50 μL PCR反应体系含10 mmol/L Tris-HCl(pH9.0), 50 mmol/L KCl, 2.5 mmol/L MgCl2, 200 μmol/L dNTP, 1 U TaqDNA聚合酶(Promega公司), 引物各10 pmol. 各反应液中加入150 ng基因组DNA.PCR反应条件如下: 96 ℃预变性10 min, 95 ℃变性30 s, 56-60 ℃退火1 min, 72 ℃延伸1 min, 循环30次, 72 ℃延伸5 min. PCR产物加1:1的加样缓冲液(980 g/L去离子甲酰胺, 25 mg/L二甲苯青, 25 mg/L溴酚蓝, 10 mmol/L EDTA), 95 ℃变性6 min, 立即置于冰中. 然后加样于100 g/L非变性聚丙烯酰胺凝胶中电泳, 80V, 4-5 h. 凝胶经固定(100 ml/L乙醇+10 ml/L硝酸10 min), 染色(0.012 mol/L硝酸银20-30 min), 显色(0.28 mol/L碳酸钠+19 mg/L甲醛)至带型清晰, 干胶仪(Bio-Rad公司)干燥, 用Flour-STM凝胶成像系统(Bio-Rad公司)扫描, Quantity-one图像分析软件分析图像. 判断标准: 参照Tomlinson et al[14], 当扩增产物为2条分开的主要条带时, 说明此位点为杂合性, 判断为信息个体. 如肿瘤条带中的一条的信号强度与其正常对照组织相应产物比较, 减弱50%以上或消失, 判断为杂合性丢失(loss of heterozygosity, LOH); 与正常对照相比, 如出现异常条带, 则判断为微卫星不稳定(microsatellite instability, MSI); 当正常对照组织的扩增产物仅为一条时, 称为非信息个体, 不能用来判断LOH.两个位点中至少有一个位点发生LOH, 则判断为SMAD4/DPC4杂合性丢失.
| 微卫星位点 | 序列 | 退火温度(℃) |
| D18S46 | F 5'-GAATAGCAGGACCTATCAAAGAGC-3' | 60 |
| R 5'-CAGATTAAGTGAAAACAGCATATGTG-3' | ||
| D18S474 | F 5'-TGGGGTGTTTACCAGCATC-3' | 56 |
| R 5'-TGGCTTTCAATGTCAGAAGG-3' |
统计学处理 应用SPSS(10.0版本)统计学软件对结果进行分析. 计数资料用x2检验, >1/5理论频数<5时, 采用确切概率法, 对等级分组资料用Kruskal-Wallis检验, 以P<0.05有统计学意义.
2.1 胃癌DPC4/SMAD4杂合性丢失
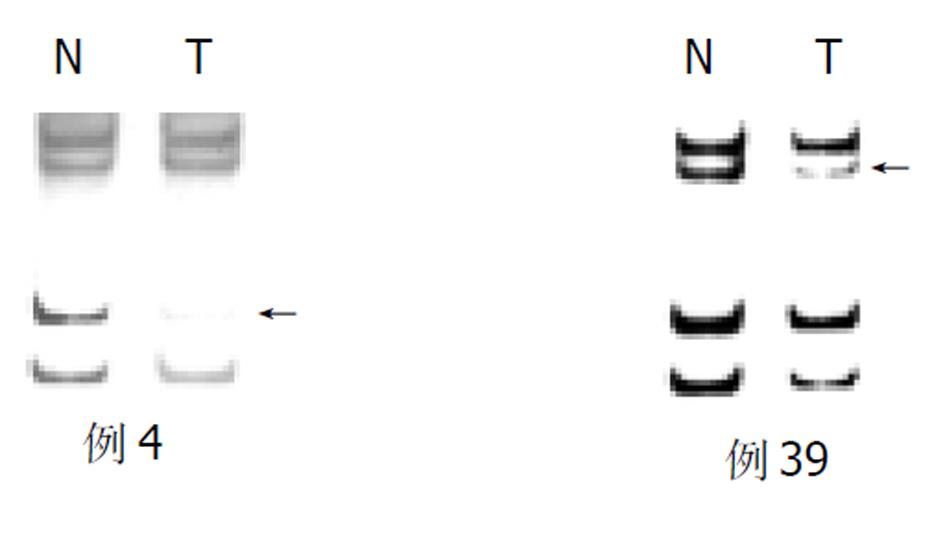
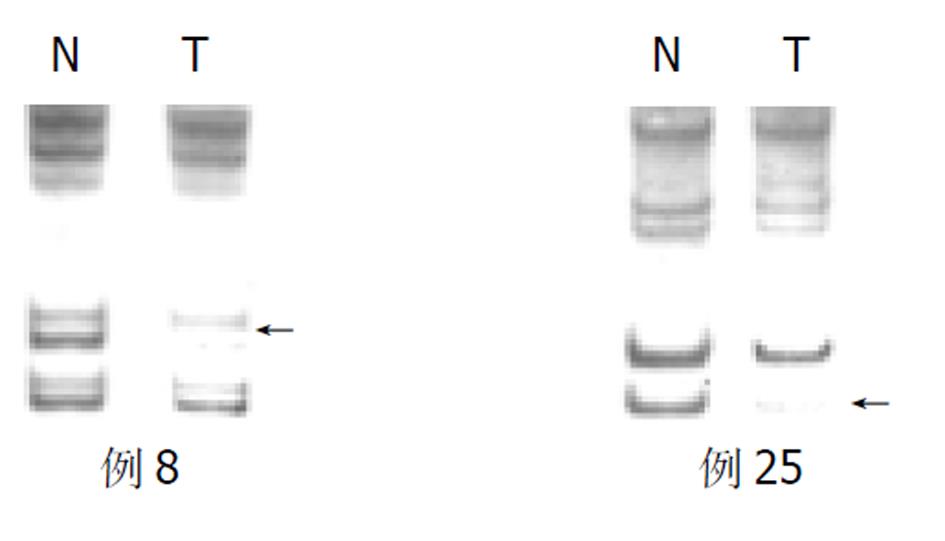
胃癌50例中, D18S46位点发生杂合性丢失的频率为36.1%, D18S474为39.1%, SMAD4/DPC4发生杂合性丢失的频率为59.2%(图1, 图2).
随着胃壁浸润深度的加深, SMAD4/DPC4 LOH的发生率从20.0%递升至37.5%, 64.5%和100.0% (P<0.05); TNMⅠ期和Ⅱ期SMAD4/DPC4 LOH为44.0%, 而TNM Ⅲ期和Ⅳ期则升至75.0% , 两组有显著性差异(P<0.05). 胃癌直径≥5 cm时, SMAD4/DPC4 LOH要明显高于胃癌<5 cm时(77.8%:88.4%, P<0.05). 在Borrmann分型中, 从隆起型到溃疡型、浸润型, SMAD4/DPC4 LOH也逐渐增高(33.3%:57.6%:100.0%, P<0.05). SMAD4/DPC4 LOH与患者的性别、年龄、肿瘤发生部位、组织学分级、Lauren分型及淋巴结是否发生转移均无统计学意义(P>0.05, 表1).
肿瘤的发生和发展是一多步骤参与的过程, 有癌基因的活化和抑癌基因的失活[15-24]. 根据Knudson"二次打击学说"[25], 许多抑癌基因的失活是在一对等位基因中, 一个突变, 另一个丢失, 或者两个均丢失. 通过杂合性丢失可判断染色体某一位点的一对等位基因有无丢失, 有助于定位及发现抑癌基因. 已有研究报道应用LOH分析胃癌中APC, MCC和DCC的LOH, 发现部分胃癌可能通过LOH病理途径发生[26]. 染色体18q LOH多见于进展期和远处转移的肿瘤, 且预后差[9,11]. 在18q21上存在着多种抑癌基因, 如SMAD4/DPC4, SMAD2和DCC. 胰腺癌中80%可见18q21的丢失, 结直肠癌中60%有18q21的丢失, 约1/3者有SMAD4/DPC4的突变[27]. 晚期和转移性结直肠癌中, SMAD4/DPC4的丢失很常见[11,28,29]. SMAD4/DPC4表达水平与一些恶性肿瘤发生发展、生物学行为及其预后有密切关系, 与阴性表达者比较, 阳性表达者肿瘤多分化好、生长速度慢、侵袭和转移能力弱, 预后较好[30-33]. 我们用与SMAD4紧密连锁的两个微卫星位点D18S46和D18S474分析原发性胃癌, 发现SMAD4/DPC4 LOH为59.2%. Powell et al[34]用与SMAD4邻近的两个微卫星位点分析35例胃腺癌, 发现SMAD4/DPC4 LOH为56%, 与我们的结果很接近. Yustein et al[35]用胃腺癌的异种移植物分析18q21的LOH, D18S474的LOH为58%, D18S46 LOH为53%, 18q 21 LOH为61%, 结果较我们为高, 这可能与他们用的标本是胃癌的移植物, 肿瘤细胞纯度高, 掺杂的正常细胞少有关.
我们结果显示SMAD4/DPC4 LOH与胃癌的胃壁浸润深度有关, 随着浸润深度的加深, LOH从T1 20.0%, T2 37.5%, 增高至T3 64.5%和T4 100.0%. Candusso et al[36]发现在肠型和混合型胃癌中, 18q21 LOH随着胃壁浸润深度的加深而增高. 我们结果还显示SMAD4/DPC4 LOH与胃癌的病理分期有关, TNMⅠ期和Ⅱ期的LOH明显低于TNM Ⅲ期和Ⅳ期(44.0%∶75.0%, P<0.05). 早期胃癌与进展期胃癌比较, 尽管无统计学意义, 但还是可看到他们的差异(20%∶63.6%, P = 0.083), 这可能与我们早期胃癌样本数量少有关. Manzoni et al[37]发现17p和18q21同时存在的杂合性丢失, 多见于Ⅲ期和Ⅳ期的进展期胃癌, 且预后差. Maitra et al[11]分析了结直肠肿瘤, 发现有远处转移的肿瘤, SMAD4/DPC4的丢失和突变明显增高, 而表达却明显降低, 认为DPC4的改变多见于进展期肿瘤. 在Borrmann分型中, 从隆起型到溃疡型、浸润型, SMAD4/DPC4 LOH也逐渐增高(P<0.05). 我们认为SMAD4/DPC4的杂合性丢失是胃癌发展的后期事件, 由于SMAD4/DPC4表达的降低, TGF-β信号通路被破坏, 肿瘤细胞从而逃逸TGF-β介导的细胞生长抑制和凋亡作用, 促进了胃癌的发生和发展.
胃癌多发生50岁以上患者, 少见于40岁以下患者, 发生40岁以下者不足10%. 有研究提示, 青年人胃癌比老年人胃癌更可能有其分子生物学背景[38]. 本组<40岁组占8%, 未发现年龄>40岁和≤40岁两胃癌组SAMD4/DPC4 LOH检出率有显著差异. 我们研究表明, SMAD4/DPC4与胃癌的TNM分期和胃壁浸润深度有关, 且肿瘤直径>5 cm的胃癌和浸润型胃癌易发生丢失, 其可能在胃癌的发展中起重要作用.
| 1. | Takebayashi S, Ogawa T, Jung KY, Muallem A, Mineta H, Fisher SG, Grenman R, Carey TE. Identification of new minimally lost regions on 18q in head and neck squamous cell carcinoma. Cancer Res. 2000;60:3397-3403. [PubMed] |
| 2. | Fung LF, Wong N, Tang N, Lau A, Wong V, Pang CP, Suen M, King W, Johnson PJ. Genetic imbalances in pT2 breast cancers of southern Chinese women. Cancer Genet Cytogenet. 2001;124:56-61. [DOI] |
| 3. | Aoyagi Y, Yokose T, Minami Y, Ochiai A, Iijima T, Morishita Y, Oda T, Fukao K, Noguchi M. Accumulation of losses of heterozygosity and multistep carcinogenesis in pulmonary adenocarcinoma. Cancer Res. 2001;61:7950-7954. [PubMed] |
| 4. | Buschges R, Bostrom J, Wolter M, Blaschke B, Weber RG, Lichter P, Collins VP, Reifenberger G. Analysis of human meningiomas for aberrations of the MADH2, MADH4, APM-1 and DCC tumor suppressor genes on the long arm of chromosome 18. Int J Cancer. 2001;92:551-554. [PubMed] [DOI] |
| 5. | Blaker H, von Herbay A, Penzel R, Gross S, Otto HF. Genetics of adenocarcinomas of the small intestine: frequent deletions at chromosome 18q and mutations of the SMAD4 gene. Oncogene. 2002;21:158-164. [PubMed] [DOI] |
| 6. | Wistuba II, Tang M, Maitra A, Alvarez H, Troncoso P, Pimentel F, Gazdar AF. Genome-wide allelotyping analysis reveals multiple sites of allelic loss in gallbladder carcinoma. Cancer Res. 2001;61:3795-3800. [PubMed] |
| 7. | Yin Z, Babaian RJ, Troncoso P, Strom SS, Spitz MR, Caudell JJ, Stein JD, Kagan J. Limiting the location of putative human prostate cancer tumor suppressor genes on chromosome 18q. Oncogene. 2001;20:2273-2280. [PubMed] [DOI] |
| 8. | Lollgen RM, Hessman O, Szabo E, Westin G, Akerstrom G. Chromosome 18 deletions are common events in classical midgut carcinoid tumors. Int J Cancer. 2001;92:812-815. [PubMed] [DOI] |
| 9. | Biankin AV, Biankin SA, Kench JG, Morey AL, Lee CS, Head DR, Eckstein RP, Hugh TB, Henshall SM, Sutherland RL. Aberrant p16(INK4A) and DPC4/Smad4 expression in intraductal papillary mucinous tumours of the pancreas is associated with invasive ductal adenocarcinoma. Gut. 2002;50:861-868. [DOI] |
| 10. | Tarafa G, Villanueva A, Farre L, Rodriguez J, Musulen E, Reyes G, Seminago R, Olmedo E, Paules AB, Peinado MA. DCC and SMAD4 alterations in human colorectal and pancreatic tumor dissemination. Oncogene. 2000;19:546-555. [PubMed] [DOI] |
| 11. | Maitra A, Molberg K, Albores-Saavedra J, Lindberg G. Loss of Dpc4 expression in colonic adenocarcinomas correlates with the presence of metastatic disease. Am J Pathol. 2000;157:1105-1111. [DOI] |
| 12. | Had ija MP, Kapitanovic S, Radosevic S, Cacev T, Mirt M, Kovacevic D, Cacev T, Hadzija M, Spaventi R, Pavelic K. Loss of heterozygosity of DPC4 tumor suppressor gene in human sporadic colon cancer. J Mol Med. 2001;79:128-132. [DOI] |
| 13. | Lo KW, Teo PM, Hui AB, To KF, Tsang YS, Chan SY, Mak KF, Lee JC, Huang DP. High resolution allelotype of microdissected primary nasopharyngeal carcinoma. Cancer Res. 2000;60:3348-3353. [PubMed] |
| 14. | Tomlinson IP, Lambros MB, Roylance RR. Loss of heterozygosity analysis: practically and conceptually flawed. Gene Chromosomes Cancer. 2002;34:349-353. [PubMed] [DOI] |
| 15. | Werner M, Becker KF, Keller G, Hofler H. Gastric adenocarcinoma: pathomorphology and molecular pathology. J Cancer Res ClinOncol. 2001;127:207-216. [DOI] |
| 18. | 张 庆, 刘 杰, 张 力, 李 青, 李 勤, 吴 汉平, 潘 伯荣, 樊 代明. 蛋白翻译起始因子C2在胃癌组织和细胞系中表达的意义. 世界华人消化杂志. 2001;9:1157-1161. |
| 23. | 杨 琳, 王 艳萍, 吴 东瑛, 张 素敏, 李 锦毅, 张 荫昌, 辛 彦. 胃印戒细胞癌与黏液腺癌生物学特征及分子病理学机制的比较研究. 世界华人消化杂志. 2002;10:516-524. |
| 25. | Knudson AG Jr. Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985;45:1437-1443. |
| 27. | Woodford-Richens KL, Rowan AJ, Gorman P, Halford S, Bicknell DC, Wasan HS, Roylance RR, Bodmer WF, Tomlinson IP. SMAD4 mutations in colorectal cancer probably occur before chromosomal instability, but after divergence of the microsatellite instability pathway. PNAS. 2001;98:9719-9723. [PubMed] [DOI] |
| 28. | Salovaara R, Roth S, Loukola A, Launonen V, Sistonen P, Avizienyte E, Kristo P, Jarvinen H, Souchelnytskyi S, Sarlomo-Rikala M. Frequent loss of SMAD4/DPC4 protein in colorectal cancers. Gut. 2002;51:56-59. [PubMed] [DOI] |
| 29. | Ohtaki N, Yamaguchi A, Goi T, Fukaya T, Takeuchi K, Katayama K, Hirose K, Urano T. Somatic alterations of the DPC4 and Madr2 genes in colorectal cancers and relationship to metastasis. Int J Oncol. 2001;18:265-270. [DOI] |
| 30. | Argani P, Shaukat A, Kaushal M, Wilentz RE, Su GH, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Differing rates of loss of DPC4 expression and of p53 overexpression among carcinomas of the proximal and distal bile ducts. Cancer. 2001;91:1332-1341. [DOI] |
| 31. | Zondervan PE, Wink J, Alers JC, IJzermans JN, Schalm SW, de Man RA, van Dekken H. Molecular cytogenetic evaluation of virus-associated and non-viral hepatocellular carcinoma: analysis of 26 carcinomas and 12 concurrent dysplasias. J Pathol. 2000;192:207-215. [DOI] |
| 32. | Yatsuoka T, Sunamura M, Furukawa T, Fukushige S, Yokoyama T, Inoue H, Shibuya K, Takeda K, Matsuno S, Horii A. Association of poor prognosis with loss of 12q, 17p, and 18q, and concordant loss of 6q/17p and 12q/18q in human pancreatic ductal adenocarcinoma. Am J Gastroenterol. 2000;95:2080-2085. [PubMed] [DOI] |
| 33. | Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, Kern SE, Hruban RH. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002-2006. [PubMed] |
| 34. | Powell SM, Harper JC, Hamilton SR, Robinson CR, Cummings OW. Inactivation of smad4 in gastric corcinomas. Cancer Res. 1997;57:4221-4224. [PubMed] |
| 35. | Yustein AS, Harper JC, Petroni GR, Cummings OW, Moskaluk CA, Powell SM. Allelotype of gastric adeno carcinoma. Cancer Res. 1999;59:1437-1441. [PubMed] |
| 36. | Candusso ME, Luinetti O, Villani L, Alberizzi P, Klersy C, Fiocca R, Ranzani GN, Solcia E. Loss of heterozygosity at 18q21 region in gastric cancer involves a number of cancer-related genes and correlates with stage and histology, but lacks independent prognostic value. J Pathol. 2002;197:44-50. [PubMed] [DOI] |