修回日期: 2002-11-20
接受日期: 2002-11-29
在线出版日期: 2003-03-15
目的: 用人源化抗人肝癌单链抗体(hscFv25)融合人突变型TNFα构建重组免疫毒素, 对荷肝癌(SMMC-7721)裸鼠进行体内杀伤活性实验.
方法: 用纯化的hscFv25-mTNFα重组免疫毒素经尾静脉注射治疗荷肝癌裸鼠, 2 wk后处死裸鼠, 观察肿瘤的体积、质量, 并对瘤组织进行TNFα的免疫组化染色.
结果: hscFv25-mTNFα治疗组对荷肝癌裸鼠的有效率为5/5, 其中2/5为完全缓解, 3/5为部分缓解, 与mTNFα对照组相比, 有显著差异(Xh2 = 6.62, P<0.05), 疗效明显高于对照组, 治疗组裸鼠的肝、肺等组织中未见转移性病灶, 经hscFv25-mTNFα治疗后的瘤组织, TNFα呈弥漫性阳性反应, 主要存在于瘤组织的胞质中.
结论: hscFv25-mTNFα对荷肝癌裸鼠具有一定的抑瘤作用, 为HCC治疗打下基础.
引文著录: 张静, 刘彦仿, 杨守京, 乔庆, 张素珍, 程虹. 人源化抗肝癌单链抗体融合突变型TNFα的导向作用. 世界华人消化杂志 2003; 11(3): 285-288
Revised: November 20, 2002
Accepted: November 29, 2002
Published online: March 15, 2003
AIM: To explore the cytotoxic effects of humanized scFv25 and the fusion to mutant TNFα on HCC xenografts in nude mice.
METHODS: The mice with HCC xenografts were injected the purified recombinant immunotoxin through tail vain and executed after two weeks. The bulk and weight of tumor were observed. Expression of TNFα was detected by immunohistochemical staining in the tumor tissues.
RESULTS: The tumor regression trials of hscFv25-mTNFα showed 5/5 effective, with 2/5 completely remission and 3/5 partly remission. The therapeutic result of hscFv25-mTNFα was better than that of mutant TNFα (Xh2 = 6.62, P < 0.05). The HCC tissue treated by hscFv25-mTNFα expressed TNFα, and the positive granules were mainly existed in cytoplasm.
CONCLUSION: Recombinant immunotoxin the hscFV25-mTNFα can regress the growth of HCC with a great potential.
- Citation: Zhang J, Liu YF, Yang SJ, Qiao Q, Zhang SZ, Cheng H. Targeting therapeutic of humanized scFv25 and the fusion to mutant TNFα against hepatocellular carcinoma: a preliminary study. Shijie Huaren Xiaohua Zazhi 2003; 11(3): 285-288
- URL: https://www.wjgnet.com/1009-3079/full/v11/i3/285.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i3.285
肝细胞肝癌(hepatocellular carcinoma, HCC)是严重危害人类健康的恶性肿瘤之一[1-5], 其诊断和治疗一直是亟待解决的重点课题之一[6-10]. 1980年代首次开始了基因工程抗体的新技术, 单链抗体是由完整的抗体可变区轻链(VL)和可变区重链(VH)组成, 中间有一条肽链相连[11,12], 目前已在当今肿瘤性疾病中显示了广泛的应用前景[13-15]. 我室与军事医学科学院合作, 成功构建了人源化抗肝癌单链抗体(hscFv25)[16], 在此基础上, 我们将之与人突变型肿瘤坏死因子(mTNFα)连接, 在对其进行诱导表达、鉴定和纯化后, 对荷肝癌(SMMC-7721)裸鼠进行了体内杀伤活性的研究.
人肝癌细胞株SMMC-7721为本室保存, RPMI 1640常规传代培养; RPMI 1640购自Gibco公司; EnVisionTM System及DAB显色剂购自Dako公司; 突变型TNFα(mTNFα)蛋白冻干粉由本校生物技术中心提供; 鼠抗人TNFα单克隆抗体购自Stanta Cruz公司; 超级小牛血清购自杭州四季青生物工程材料研究所. 裸鼠Balb/c-nu15只, 分别购自本校动物实验中心及上海细胞所, 并一直在本校动物实验中心无特殊病原菌(SPF三级动物饲养条件)实验室进行饲养及实验研究. 消化对数生长期的SMMC-7721细胞, 无血清RPMI 1 640培养液洗涤2次, 倒置显微镜下计算活细胞数, 调整细胞数后, 将细胞重悬于PBS中, 在无菌条件下, 对15只体质量为17-24 g的裸鼠, 在右侧背部按2×106/只, 进行接种, 定期观察肿瘤生长情况.
scFv25-mTNFα 的导向作用. 荷癌裸鼠15只, 肿瘤直径约2.0 mm, 随机分3组, 每组5只, 第1组为阴性对照组, 给予PBS; 第2组为阳性对照组, 给予mTNFα; 第3组为实验组, 给予hscFv25-mTNFα. 给药方式为尾静脉注射, 实验组给药剂量为80 μL(2g/L)/只, 相当于mTNFα 6 μg/只, 阳性对照组给予mTNFα的剂量为12 μg/只, 1次/d. 1 wk为1疗程, 2 wk后处死各组荷癌裸鼠, 取出瘤组织称重、测量体积后, 用40 g/L中性甲醛进行固定, 制备石蜡切片备用, 并进行常规HE染色. 对各组肿瘤组织用鼠抗人TNFα 单克隆抗体进行免疫组化染色. 石蜡切片常规脱蜡至水, 用800 mL/L甲醇H2O2(甲醇4 mL +三蒸水1mL + H2O2 50 mL)阻断内源性过氧化物酶, RT 40 min; PBS振洗5 min×2次; 加入鼠抗人TNFα单克隆抗体, 4℃过夜, 次日37℃孵育30 min, PBS振洗5min×3次; 滴加EnVisionTM孵育1 h, PBS振洗5 min×3次; DAB-H2O2显色5-10 min; 蒸馏水漂洗, 脱水透明, DPX封固. 实验中设立无关抗出血热病毒IB3抗体对照、不加抗体的阴性对照.
统计学处理 采用Xh2检验, P<0.05为显著差异, P<0.01为非常显著, P>0.05为无差异.
体质量16-24 g的裸鼠15只, 分别在其右侧背部接种2×106 SMMC-7721细胞(PBS悬浮), 7 d即可见到15只裸鼠都有肿瘤生长, 从10 d开始给药.
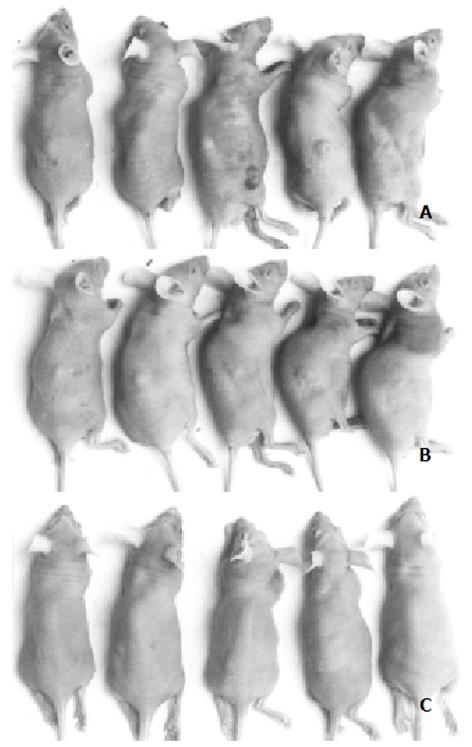
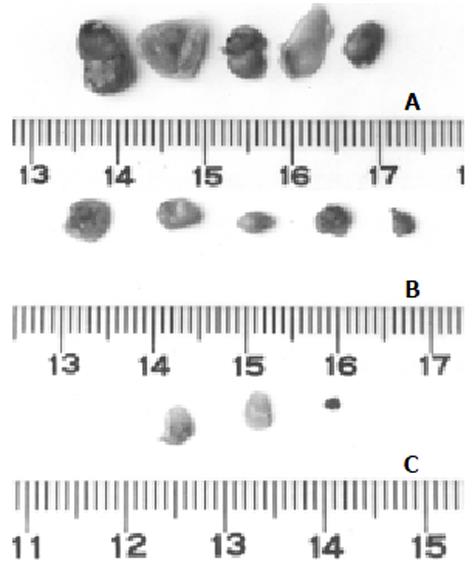
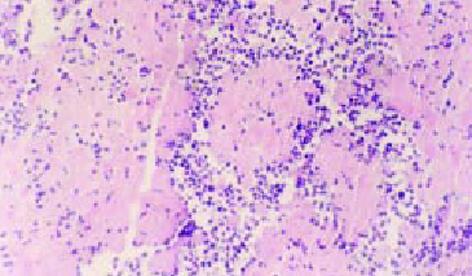
各组荷肝癌裸鼠经抗肝癌重组免疫毒素治疗14 d后的裸鼠情况及肿瘤组织见图1, 2, 各实验组荷肝癌裸鼠肿瘤体积、肿瘤质量及治疗结果见表1. hscFv25-mTNFα治疗组的有效率为5/5, 其中为2/5完全缓解, 3/5为部分缓解. mTNFα对照组的有效率为5/5, 均为部分缓解, 其肿瘤质量抑制率与hscFv25-mTNFα治疗组相比, 有显著差异(Xh2 = 6.62, P<0.05), 疗效明显低于治疗组. 对实验组肝、肺等组织进行连续切片、染色, 都没有见到转移性病灶, 完全缓解的荷肝癌裸鼠皮下组织经连续切片、染色证实未见残留的肿瘤细胞, 部分缓解的荷肝癌裸鼠的肿瘤组织, 经HE染色后, 可见瘤组织中存在大量坏死区(图3).
| 处理 | 瘤体积(mm×mm) | 瘤质量(mg) | 瘤体积抑制率(%) | 瘤质量抑制率(%) | 疗效 |
| PBS 1 | 9×5 | 119 | 0 | 0 | NR |
| 2 | 7×7 | 87 | 0 | 0 | NR |
| 3 | 9×4 | 51 | 0 | 0 | NR |
| 4 | 5×3 | 26 | 0 | 0 | NR |
| 5 | 5×4 | 30 | 0 | 0 | NR |
| hscFv25 1 | 0.8×0.5 | 2 | 99.2 | 96.8 | PR |
| -mTNFα 2 | 2×3 | 10 | 81.8 | 84.0 | PR |
| 3 | 2×3 | 15 | 81.8 | 76.0 | PR |
| 4 | 0 | 0 | 100 | 100 | CR |
| 5 | 0 | 0 | 100 | 100 | CR |
| mTNFα 1 | 2×2 | 11 | 87.9 | 82.4 | PR |
| 2 | 3×3 | 22 | 72.7 | 64.9 | PR |
| 3 | 2×4 | 15 | 75.8 | 76.0 | PR |
| 4 | 2×3 | 10 | 81.8 | 84.0 | PR |
| 5 | 2×3 | 10 | 81.8 | 84.0 | PR |
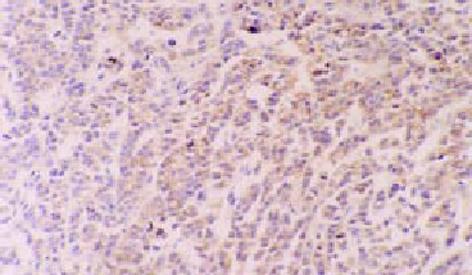
以鼠抗人TNFα单克隆抗体为一抗, 对瘤组织进行免疫组化染色, 结果可见经hscFv25-mTNFα治疗后的瘤组织呈弥漫性阳性反应, 阳性颗粒为棕黄色, 主要存在于瘤细胞的胞质中(图4). 无关抗出血热IB3抗体为阴性反应, 未给予TNFα治疗的PBS对照组的瘤组织为阴性或弱阳性反应.
近20年来, 抗体已通过了多克隆抗体→单克隆抗体→基因工程抗体三个阶段的发展, 其中基因工程抗体因其显著的优点, 已成为众多学者关注的热点, 目前抗肿瘤的单链抗体已有进入I期临床的报道[17,18], scFv具有分子量小、特异性强、亲合力较高等优点[19-22], 在其基因3'端接上毒素、酶、细胞因子等, 构成重组免疫毒素, 就可以作为良好的导弹, 对肿瘤细胞发挥杀伤作用[22-25]. HAb25是我室自行建立的抗人肝癌单克隆抗体, 在此基础上构建的抗肝癌单链抗体hscFv25经实验证明, 也具有较好的导向作用[26], 因此我们将其与高效、低毒性的mTNFα融合, 构建了hscFv25-mTNFα原核表达载体PGEX4T-1/hscFv25-mTNFα, 通过转化大肠杆菌JM109后, 通过IPTG诱导进行了目的蛋白的初步表达[27], 表达的蛋白产物经体外分离、变性、复性及纯化后, 采用SDS聚丙烯酰胺凝胶电泳、Western blot进行分析、鉴定证实后, 对荷肝癌裸鼠进行体内杀伤活性实验.
TNFα是由单核/巨噬细胞分泌的一种蛋白质, 对大多数肿瘤细胞具有特异性的细胞毒作用, 但对正常细胞和某些肿瘤细胞却没有影响, 其抗肿瘤作用比较复杂, 普遍认为可能与以下几种机制有关[28-31]: 直接杀伤肿瘤细胞; 刺激产生肿瘤特异性的细胞毒抗体; 阻断肿瘤血液供应; 促进肿瘤细胞凋亡. 尽管TNFα是迄今为止所发现的直接杀伤肿瘤作用最强的一种生物活性物质, 但毒副作用十分严重, 事实上, TNFα的氨基酸序列和恶液质素完全一样, 恶液质素可以抑制脂蛋白脂肪酶的活性, 引起全身衰竭, 在临床上出现发热、寒战、恶心、呕吐, 严重者甚至出现全身消瘦, 导致各脏器衰竭而死亡, TNFα全身治疗的效果极不理想, 目前TNFα在临床治疗中仅为局部使用[32]. 文献研究通过采用定点突变或聚合物改造TNF分子可以降低其活性[33,34], 例如(J Biol Response Mod, 1988; 7: 587-589)分别在TNFα的N端增加三个氨基酸、在TNFα的第四个外显子编码序列的N端增加三个氨基酸, 研制出新型具有更强、更广泛的抗癌活性, 且毒性反应也大大降低的TNF-S. (Int J Cancer, 1991; 48: 744-748)将TNFα去掉7个N端氨基酸同时将Pro8Ser9Asp10改为ArgLysArg, 形成TNFα突变体, 体外抗肿瘤活性可提高7倍, 且与TNFR的亲和力提高, 毒性比天然TNFα降低18倍, 而(Zhonghua Weishengwu Mianyixue Zazhi, 1996; 16: 254-258)在此基础上, 又同时将C端第157位Leu由Phe替代, 与天然野生型TNFα相比, 细胞毒活性提高约两个数量级, 从而显示了潜在的临床应用价值. 在本实验中, 我们选用的TNFα突变体即为此种.
通过实验, 我们认为hscFv25-mTNFα具有较好的导向杀伤作用, 疗效明显高于对照组, 相比实验组的m/hscFv25-mTNFα的用量(6 mg/只), 对照组mTNFα的用量达到12 mg/只, 这提示在scFv25的导向下, mTNFα很可能会更有效地集中在肿瘤组织的局部, 从而发挥杀瘤作用. 同时对瘤组织进行用鼠抗TNFα单克隆抗体免疫组化染色, 结果显示: 瘤细胞中有弥漫性、强弱不等的TNFα分布, 说明scFv25-mTNFα在瘤组织内具有较好的分布率, 说明hscFv25-mTNFα是有一定潜能的抗肝癌基因工程抗体, 为今后的工作打下了基础.
编辑: N/A
| 1. | Luo YQ, Wu MC, Cong WM. Gene expression of hepatocyte growth factor and its receptor in HCC and nontumorous liver tissues. World J Gastroenterol. 1999;5:119-121. [PubMed] [DOI] |
| 2. | Qian QJ, Xue HB, Qu ZQ, Fang SG, Cao HF, Wu MC. In situ detection of tumor infiltrating lymphocytes expressing perforin and fas ligand genes in human HCC. World J Gastroenterol. 1999;5:12-14. [PubMed] [DOI] |
| 3. | Guo XZ, Shao XD, Xu JH, Zhao JJ, Li HY, Wang D. Expression of bcl-xL mRNA in hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2002;10:530-532. [DOI] |
| 4. | Yang YL, Dou KF, Li KZ. Correlation of UPAR and VEGF expression with invasion and metastasis in human hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2002;10:381-383. [DOI] |
| 5. | Zeng JX, Wang WL, Luo WJ, Wang ZL. Construction of differentially expressed cDNA library in human hepatocellular carcinoma apoptotic cells with suppression subtractive hybridization. Shijie Huaren Xiaohua Zazhi. 2001;9:1233-1237. [DOI] |
| 6. | Cao W, Wang ZM, Liang ZH, Zhang HX, Wang YQ, Guan Y, Li WX, Pan BR. Effects of angiogenesis inhibitor TNP-470 with lipiodol in arterial embolization of liver cancer in rabbits. Shijie Huaren Xiaohua Zazhi. 2000;8:629-632. [DOI] |
| 7. | Qi YY, Zhou LG, Wang WX, Chen WJ, Xiang KL, Zhao XY, Yang TH, Yi XZ. Duai-phase enhanced spiral CT in the diagnosis of hepatocellular carcinoma with tumor thrombus in portal vein. Shijie Huaren Xiaohua Zazhi. 2002;10:384-387. [DOI] |
| 8. | Tian XM, Zhang ZX. The anticancer activity of resveratrol on implanted tumor of HepG2 in nude mice. Shijie Huaren Xiaohua Zazhi. 2001;9:161-164. [DOI] |
| 9. | Qu B, Li BJ, Lu ZW, Pan HL. Clinical significance of telomerase activity detected in fine-needle aspiration specimens to liver cancer diagnosis. Shijie Huaren Xiaohua Zazhi. 2001;9:538-541. [DOI] |
| 10. | Zhen JY, Li KZ, Wang WZ. Impact of the expression of P27KIPI on apoptosis and progression of hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2002;10:883-886. [DOI] |
| 11. | Colcher D, Pavlinkova G, Beresford G, Booth BJ, Batra SK. Single-chain antibodies in pancreatic cancer. Ann N Y Acad Sci. 1999;880:263-280. [PubMed] [DOI] |
| 12. | zu Putlitz J, Skerra A, Wands JR. Intracellular expression of a cloned antibody fragment interferes with hepatitis B virus surface antigen secretion. Biochem Biophys Res Commun. 1999;255:785-791. [PubMed] [DOI] |
| 13. | Pavlinkova G, Beresford GW, Booth BJ, Batra SK, Colcher D. Pharmacokinetics and biodistribution of engineered single-chain antibody constructs of MAb CC49 in colon carcinoma xenografts. J Nucl Med. 1999;40:1536-1546. [PubMed] |
| 14. | Pavlinkova G, Booth BJ, Batra SK, Colcher D. Radioimmunotherapy of human colon cancer xenografts using a dimeric single-chain Fv antibody construct. Clin Cancer Res. 1999;5:2613-2619. [PubMed] |
| 15. | Goel A, Colcher D, Baranowska-Kortylewicz J, Augustine S, Booth BJ, Pavlinkova G, Batra SK. Genetically engineered tetravalent single-chain Fv of the pancarcinoma monoclonal antibody CC49: improved biodistribution and potential for therapeutic application. Cancer Res. 2000;60:6964-6971. [PubMed] |
| 16. | Yuan QA, Yu WY, Huang CF. [Construction and expression of a hepatocellular carcinoma specific rodent and its humanized single-chain Fv fragments in Escherichia coli]. Sheng Wu Gong Cheng Xue Bao. 2000;16:86-90. [PubMed] |
| 17. | Turatti F, Mezzanzanica D, Nardini E, Luison E, Maffioli L, Bambardieri E, de Lalla C, Canevari S, Figini M. Production and validation of the pharmacokinetics of a single-chain Fv fragment of the MGR6 antibody for targeting of tumors expressing HER-2. Cancer Immunol Immunother. 2001;49:679-686. [PubMed] [DOI] |
| 18. | Mayer A, Tsiompanou E, O'Malley D, Boxer GM, Bhatia J, Flynn AA, Chester KA, Davidson BR, Lewis AA, Winslet MC. Radioimmunoguided surgery in colorectal cancer using a genetically engineered anti-CEA single-chain Fv antibody. Clin Cancer Res. 2000;6:1711-1719. [PubMed] |
| 19. | Niv R, Assaraf YG, Segal D, Pirak E, Reiter Y. Targeting multidrug resistant tumor cells with a recombinant single-chain FV fragment directed to P-glycoprotein. Int J Cancer. 2001;94:864-872. [PubMed] [DOI] |
| 20. | McCall AM, Shahied L, Amoroso AR, Horak EM, Simmons HH, Nielson U, Adams GP, Schier R, Marks JD, Weiner LM. Increasing the affinity for tumor antigen enhances bispecific antibody cytotoxicity. J Immunol. 2001;166:6112-6117. [PubMed] [DOI] |
| 21. | Cooke SP, Boxer GM, Lawrence L, Pedley RB, Spencer DI, Begent RH, Chester KA. A strategy for antitumor vascular therapy by targeting the vascular endothelial growth factor: receptor complex. Cancer Res. 2001;61:3653-3659. [PubMed] |
| 22. | van der Poel HG, Molenaar B, van Beusechem VW, Haisma HJ, Rodriguez R, Curiel DT, Gerritsen WR. Epidermal growth factor receptor targeting of replication competent adenovirus enhances cytotoxicity in bladder cancer. J Urol. 2002;168:266-272. [PubMed] [DOI] |
| 23. | Goel A, Baranowska-Kortylewicz J, Hinrichs SH, Wisecarver J, Pavlinkova G, Augustine S, Colcher D, Booth BJ, Batra SK. 99mTc-labeled divalent and tetravalent CC49 single-chain Fv's: novel imaging agents for rapid in vivo localization of human colon carcinoma. J Nucl Med. 2001;42:1519-1527. [PubMed] |
| 24. | Matsumoto H, Liao S, Arakawa F, Ueno A, Abe H, Awasthi A, Kuroki M, Kuroki M. Targeting of interleukin-2 to human MK-1-expressing carcinoma by fusion with a single-chain Fv of anti-MK-1 antibody. Anticancer Res. 2002;22:2001-2007. [PubMed] |
| 25. | Akamatsu Y, Murphy JC, Nolan KF, Thomas P, Kreitman RJ, Leung SO, Junghans RP. A single-chain immunotoxin against carcinoembryonic antigen that suppresses growth of colorectal carcinoma cells. Clin Cancer Res. 1998;4:2825-2832. [PubMed] |
| 26. | Cheng H, Liu YF, Zhang HZ, Shen WA. Construction of anti-HCC bifunctional antibody retroviridae vector and virus packed cell. Shijie Huaren Xiaohua Zazhi. 2000;8:708-709. [DOI] |
| 27. | Zhang J, Liu YF, Yang SJ, Sun ZW, Qiao Q, Zhang SZ. Construction and expression of mouse/humanized scFv and their fusion to humanized mutant TNF-a against hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:616-620. [DOI] |
| 28. | Vaskivuo TE, Stenbäck F, Tapanainen JS. Apoptosis and apoptosis-related factors Bcl-2, Bax, tumor necrosis factor-alpha, and NF-kappaB in human endometrial hyperplasia and carcinoma. Cancer. 2002;95:1463-1471. [PubMed] [DOI] |
| 29. | Inoue H, Shiraki K, Yamanaka T, Ohmori S, Sakai T, Deguchi M, Okano H, Murata K, Sugimoto K, Nakano T. Functional expression of tumor necrosis factor-related apoptosis-inducing ligand in human colonic adenocarcinoma cells. Lab Invest. 2002;82:1111-1119. [PubMed] [DOI] |
| 30. | Kandasamy K, Srivastava RK. Role of the phosphatidylinositol 3'-kinase/PTEN/Akt kinase pathway in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in non-small cell lung cancer cells. Cancer Res. 2002;62:4929-4937. [PubMed] |
| 31. | Vaculová A, Hofmanova J, Soucek K, Kovariková M, Kozubík A. Tumor necrosis factor-alpha induces apoptosis associated with poly(ADP-ribose) polymerase cleavage in HT-29 colon cancer cells. Anticancer Res. 2002;22:1635-1639. [PubMed] |
| 32. | Thomas PS, Heywood G. Effects of inhaled tumour necrosis factor alpha in subjects with mild asthma. Thorax. 2002;57:774-778. [PubMed] [DOI] |
| 33. | Yamamoto M, Oshiro S, Tsugu H, Hirakawa K, Ikeda K, Soma G, Fukushima T. Treatment of recurrent malignant supratentorial astrocytomas with carboplatin and etoposide combined with recombinant mutant human tumor necrosis factor-alpha. Anticancer Res. 2002;22:2447-2453. [PubMed] |
| 34. | Terlikowski SJ. Local immunotherapy with rhTNF-alpha mutein induces strong antitumor activity without overt toxicity--a review. Toxicology. 2002;174:143-152. [PubMed] [DOI] |