修回日期: 2002-08-02
接受日期: 2002-08-16
在线出版日期: 2003-03-15
目的: 观察三氧化二砷对体外培养的人肝癌细胞株细胞端粒酶表达和细胞生长抑制的影响及诱导细胞凋亡的作用, 探讨三氧化二砷的抗肿瘤作用及机制.
方法: 采用噻唑蓝比色分析法(MTT法), 流式细胞分析, 原位杂交方法以及TUNEL原位末端标记法观察和检测了不同浓度的三氧化二砷对人肝癌细胞株(SMMC-7721)细胞的增生, 细胞DNA含量的分布, 细胞端粒酶表达的影响及细胞凋亡的诱导作用.
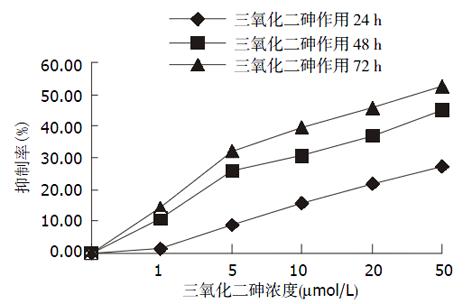
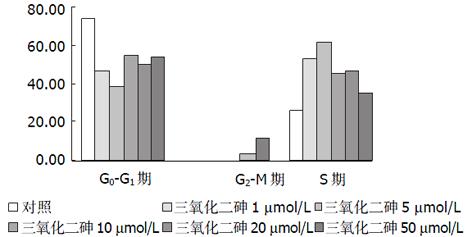
结果: 20, 5, 5 μmol/L三氧化二砷在24, 48, 72 h时抑制率分别为22.0%, 25.7%, 32.0%, 均能明显抑制人肝癌细胞株细胞的增生(单侧置信区间为10.1, 23.7, 18.0, P<0.05). 流式细胞分析显示加药组G0/G1期细胞比例减少, S期细胞所占比例增高. 随着药物浓度的增加细胞端粒酶的表达下降, 而凋亡细胞数增加.
结论: 三氧化二砷能抑制人肝癌细胞增生, 其抑制作用具有时间-计量效应关系, 并能抑制肝癌细胞端粒酶的表达及诱导肝癌细胞凋亡.
引文著录: 苏颖, 陈增, 林可焴, 邹长棪, 林华妹. 三氧化二砷抑制肝癌细胞端粒酶表达及诱导细胞凋亡作用. 世界华人消化杂志 2003; 11(3): 264-267
Revised: August 2, 2002
Accepted: August 16, 2002
Published online: March 15, 2003
AIM: To investigate the effect of arsenic trioxide on expression of telomerase and induction of apoptosis of hepatoma cells in vitro.
METHODS: The human hepatoma cells were incubated with variety concentration of arsenic trioxide. The cell growth and proliferation were detected by MTT assay. DNA distribution in cell was analyzed with flow cytometry. The expression of telomerase was studied by in situ hybridization, and induction of apoptosis was observed with terminal deoxynucleotidy transferase nick and labeling (TUNEL).
RESULTS: Inhibitory rate of proliferation of hepatoma cell with arsenic trioxide at 24, 48, 72 hours was 22.0%, 25.7%, 32.0%, respectively (P < 0.05). Flow cytometry study indicated that ratio of G0/G1 phase cell was decreased and the portion of S phase cell increased following treatment with arsenic trioxide. The expression of telomerase was decreased and the number of apoptotic cells increased with the increment of arsenic trioxide dosage.
CONCLUSION: Arsenic trioxide may inhibit the proliferation of hepatoma cells in a time-and concentration-dependent manner, and suppress the expression of telomerase and induce apoptosis of human hepatoma cells.
- Citation: Su Y, Chen Z, Lin KY, Zou CY, Lin HM. Effect of arsenic trioxide on expression of telomerase and induction of apoptosis of human hepatoma cell. Shijie Huaren Xiaohua Zazhi 2003; 11(3): 264-267
- URL: https://www.wjgnet.com/1009-3079/full/v11/i3/264.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i3.264
端粒参与DNA复制, 染色体定位和染色体稳定, 在控制细胞生长寿命等方面起重要作用[1,2]. 端粒酶是一种能够维持端粒长度的酶, 可不断合成端粒重复序列添加至染色体末端, 而使细胞具有永生化的生物学特性. 近来研究表明, 端粒酶的激活及表达与恶性肿瘤的发生, 发展存在密切关系[3-6]. 我们研究了三氧化二砷(As2O3)对人肝癌细胞株(SMMC-7721)细胞端粒酶表达和细胞生长的影响及诱导细胞凋亡的作用, 并探讨二者的关系.
人肝癌细胞株SMMC-7721由成都华西医科大学彭文珍老师惠赠. 细胞呈贴壁生长, 常规培养于含150 mL/L新生牛血清的RPMI1 640完全培养液中, 置37℃, 50 mL/L CO2孵箱中培养、传代, 每2-3 d传代1次. 1 g/L As2O3 (哈尔滨医科大学附属第一医院). 噻唑蓝(MTT, Fluka公司). 端粒酶原位杂交检测试剂盒(北京大学医学部病理系). 原位细胞凋亡检测(TUNEL)试剂盒(宝灵曼公司). 流式细胞DNA试剂盒(DNA-Prep Reagents kit)(Coulter公司).
采用MTT法检测As2O3作用后对SMMC-7721细胞增生的抑制作用. 收集对数生长期细胞, 以1×104细胞/孔浓度种植于96孔板, 加入不同浓度的As2O3, 对照组加入等量的培养液, 每组设3个复孔, 37℃, 50 μL/L CO℃培养 24, 48, 72 h, 每孔加入2 g /L MTT 50 mL, 继续孵育4 h, 加入150 m二甲基亚砜(DMSO), 振荡溶解10 min, 酶标仪以波长570 nm测A值. 对数生长期细胞以1×109细胞/ L浓度种植于培养瓶, 每瓶细胞数约200万, 细胞贴壁后加入不同浓度的As2O3, 对照组加入等量培养液, 继续培养24 h. 收集细胞, PBS洗2次, 滴片, 室温下干燥, 按试剂盒说明书, 采用原位杂交方法检测细胞端粒酶 mRNA的表达. 以未加药者作阴性对照, 以杂交稀释液代替生物素探针杂交液作空白对照. 计数300个细胞, 计算阳性细胞比率. 细胞凋亡的检测采用TUNEL原位末端标记法. 收集经药物处理的细胞及对照组细胞, 滴片, 按试剂盒说明书进行TUNEL原位末端标记, 以未加药者作阴性对照, 以标记溶液代替TUNEL反应混合液作空白对照, 以人外周血淋巴细胞滴片作阳性对照, 计数300个细胞, 计算凋亡指数(AI), AI = (凋亡细胞数/总细胞数)×100%. 收集经不同浓度As2O3作用的细胞和对照组细胞, 取1×106个细胞, 冷PBS(pH7.2)漂洗2次, 过滤, 加入DNA-prep Reagent, 置4℃冰箱过夜, 流式细胞仪(BI0-Rad公司)检测, 数据由ModFit软件分析.
统计学处理 采用t检验进行显著性检验.
As2O3对人肝癌细胞株细胞有显著的抑制增生作用, 且抑制作用随药物浓度提高、作用时间延长而增强(图1). 同阴性对照比较有统计学意义(P<0.05, 表1).
| 药物作用 | As2O3 (μmol/L) | |||||
| 对照 | 1.00 | 5.00 | 10.00 | 20.00 | 50.00 | |
| 增生抑制 | ||||||
| 24 h | 0.0 | 1.2±10.8 | 8.9±10.5 | 15.9±8.5 | 22.0±5.0a | 27.3±7.8a |
| 48 h | 0.0 | 10.6±5.8 | 25.7±0.9a | 30.9±1.7b | 36.9±2.0b | 45.3±2.2 |
| 72 h | 0.0 | 14.4±12.6 | 32.0±5.9a | 39.6±0.9b | 45.4±2.6b | 52.2±4.9b |
| 凋亡指数 | 26.7±2.1 | 36.0±5.2 | 35.3±2.3 | 43.0±7.0a | 47.7±8.5a | 50.3±7.5b |
| 端粒酶表达 | 96.3±0.6 | 73.0±14.9 | 72.7±8.7a | 72.0±5.3b | 64.3±7.5b | 41.0±0.1c |
As2O3抑制肝癌细胞端粒酶的表达, 当浓度为5 μmol/L对端粒酶的表达即有明显抑制作用, 并随着浓度的提高抑制作用增强(表1).
As2O3诱导肝癌细胞的调亡, 调亡细胞数随药物浓度的升高而增多(表1).
研究表明85%-95%的恶性肿瘤细胞端粒酶阳性[7,8], 而正常的体细胞几乎阴性[9-11], 端粒酶的活化可能是各种基因改变导致肿瘤细胞绕过无限增生的限制获得永生化的共同通路, 是肿瘤细胞克隆繁衍, 使癌症得以发展的重要因素[12,13]. 因此, 如何调节端粒酶的活性成为治疗肿瘤的一个新的探索途径, 以限制端粒酶活性为目标的肿瘤治疗可能具有更广阔的临床应用前景[14-18]. 而细胞凋亡不仅在维护机体内环境稳定的方面起重要作用, 并与肿瘤的发生、发展、消退及转归有密切关系[19]. 诱导肿瘤细胞凋亡已成为肿瘤治疗的新思路, 可能是寻找抗肿瘤药物的新靶点[20-22].
砷剂及其化合物是一种剧毒物, 有致癌及致畸作用, 但砷剂也有其有益的生物学作用, 与其他微量元素一样, 低浓度时对人体有益, 并且有抗癌作用[23]. 我国学者应用砷化合物(As2O3)治疗急性早幼粒白血病(APL)获得满意疗效, 进一步研究证明砷剂通过诱导APL细胞凋亡而发挥作用. 近年来As2O3在实体瘤的研究也逐渐增多, 砷剂的抗肿瘤作用与抑制细胞生长和诱导细胞凋亡有关[24-27]. 由于端粒酶与肿瘤生长及发展关系密切, 我们研究了As2O3对肝癌端粒酶表达的作用, 并探讨与As2O3其他抗肿瘤机制间的关系. 结果提示, As2O3具有下调肝癌细胞端粒酶表达的作用. As2O3对体外培养的肝癌细胞有显著的抑制增生作用, 且有时间和浓度依赖性, 这与多位作者的研究报道是一致的[28,29]. 进一步的研究表明, As2O3在抑制肝癌细胞生长的同时, 肝癌细胞端粒酶的表达下降并与剂量有效应关系, 因此端粒酶可能是As2O3的作用靶点. 另方面据近来的研究, As2O3有诱导肝癌细胞凋亡的作用, 并认为诱导凋亡是As2O3抑制肝癌细胞增生, 杀灭肝癌细胞的主要机制, As2O3可能通过改变bcl-2, Bax的表达以及两者之间的比率而诱导凋亡[30,31]. 细胞凋亡受到多种基因的调控如P53, c-myc, bcl-2, Bax等[19], 而bcl-2是最早发现与凋亡有关的基因, 是一种抗凋亡基因, Bax是bcl-2 家族成员但作用相反, Bax既可形成同聚体, 又可与bcl-2形成异二聚体, Bax蛋白与bcl-2蛋白的比值影响着细胞受到刺激后发生凋亡的比率, 上调Bax和bcl-2表达下降可诱发细胞凋亡[32-34]. Mandal et al将bcl-2基因转染Hela细胞和人结肠DiFi细胞系, 发现转染阳性的细胞端粒酶活性分别升高5-10倍和8-12倍, 说明bcl-2蛋白的过度表达是端粒酶激活的重要途径之一. 而下调bcl-2, c-myc基因的表达一方面增加凋亡比率, 另方面降低端粒酶活性[28,30]. 我们的研究也观察到As2O3在抑制细胞增生及端粒酶表达的同时, 也诱导细胞凋亡, 但其引起端粒酶表达明显下调所需的浓度低于引起调亡指数(AI)明显增高所需的浓度, 提示As2O3抑制端粒酶表达可能出现在凋亡之前, 而不是凋亡的结果, 是否为As2O3对端粒酶活性的抑制而启动凋亡过程有待进一步探讨.
As2O3作用的细胞周期动力学机制可能为周期特异性药物[23,26,28], 本组实验结果亦显示As2O3改变肝癌细胞的周期分布, 使S期细胞所占比例增高, 并有G2-M期细胞. 端粒酶活性的调节亦具有细胞周期依赖性[4], As2O3对肝癌细胞周期的影响与下调端粒酶表达的关系有待进一步研究. 以抑制端粒酶活性为靶点的研究, 除了反义核苷酸、肽核酸、核酶、苷核类似物外, 细胞分化诱导剂, 细胞周期调节剂, 其他细胞内外因子及药物, 如巯基酶及蛋白激酶抑制剂, DNA损伤因子等抑制剂都呈现了抑制端粒酶活性的作用[35-37]. As2O3具有干扰DNA及RNA合成, 抑制巯基酶, 影响细胞周期分布, 诱导细胞分化等作用[23,38,39], 这些作用可能为诱导细胞凋亡的机制, 也可能通过这些机制抑制端粒酶的活性. 总之As2O3具有抑制肝癌细胞端粒酶活性及诱导细胞凋亡的作用, 其作用机制及其端粒酶活性抑制与凋亡诱导间关系有待进一步研究.
目前, 干预端粒酶活性及诱导细胞凋亡均为肿瘤治疗研究的热点. As2O3等药物可能通过这些作用靶点而发挥抗肿瘤作用, 因此具有临床应用前景, 值得进一步研究.
编辑: N/A
| 3. | Chen B, Liu WW, Fang DC. Overview of telomerase studies. Shijie Huaren Xiaohua Zazhi. 2001;9:441-446. [DOI] |
| 4. | Wang W, Liu JP. Telomerase and tumors. Shijie Huaren Xiaohua Zazhi. 2002;10:683-688. [DOI] |
| 5. | Fu JM, Yu XF, Shao YF. Telomerase and primary liver Cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:461-463. [DOI] |
| 6. | Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701-712. [PubMed] [DOI] |
| 7. | Zhu R, Xiao WD, Xiong YY, Zhou DD, Wang L, Chen XY. The expression of telomerase and nm 23 in gastric adenocarcinoma and its prognosis. Shijie Huaren Xiaohua Zazhi. 2000;8:1305-1306. [DOI] |
| 8. | Fan GR, Wang Y, Wu GH, Huang SL. Expression of telomerase activity in primary liver carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:573. [DOI] |
| 9. | Meyerson M. Role of telomerase in normal and cancer cells. J Clin Oncol. 2000;18:2626-2634. [PubMed] [DOI] |
| 10. | Wang W, Luo HS, Yu BP. Telomerase and colorectal cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:800-802. [DOI] |
| 11. | Lin H, Chen HX, Li SG, Zeng H, Liu JH, Tong ZY. The expression of telomerase activity in various gastric mucosa lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:585-586. [DOI] |
| 12. | Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677-685. [PubMed] [DOI] |
| 13. | Rahat MA, Lahat N, Gazawi H, Resnick MB, Sova Y, Ben-Ari G, Cohen M, Stein A. Telomerase activity in patients with transitional cell carcinoma: a preliminary study. Cancer. 1999;85:919-924. [PubMed] [DOI] |
| 14. | Guo Z, Yang SM. New anti-cancer target point: progress in the studies of telomere and its inhibitors. Shijie Huaren Xiaohua Zazhi. 1999;7:607-609. [DOI] |
| 15. | de Lange T, Jacks T. For better or worse? Telomerase inhibition and cancer. Cell. 1999;98:273-275. [PubMed] [DOI] |
| 16. | Yang JL, Fang DC, Yang SM, Luo YH, Lu R, Luo KL, Liu WW. Construction of sense and antisense hTR eukaryotic expression vector. Shijie Huaren Xiaohua Zazhi. 2000;8:491-493. [DOI] |
| 17. | Zhang FX, Zhang XY, Fan DM, Deng ZY, Yan Y, Wu HP, Fan JJ. Antisense telomerase RNA induced human gastric cancer cell apoptosis. World J Gastroenterol. 2000;6:430-432. [PubMed] [DOI] |
| 18. | Izbicka E, Wheelhouse RT, Raymond E, Davidson KK, Lawrence RA, Sun D, Windle BE, Hurley LH, Von Hoff DD. Effects of cationic porphyrins as G-quadruplex interactive agents in human tumor cells. Cancer Res. 1999;59:639-644. [PubMed] |
| 19. | Shen YF, Zhuang H, Shen JW, Chen SB. Apoptosis and neoplasms. Shijie Huaren Xiaohua Zazhi. 1999;7:267-268. [DOI] |
| 20. | Gao H, Chen JY, Yu XL. Drug-induced apoptosis in treatment of liver cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:686-688. [DOI] |
| 21. | Xue XC, Fang GE, Hua JD. Gastric cancer and apoptosis. Shijie Huaren Xiaohua Zazhi. 1999;7:359-361. [DOI] |
| 22. | Gao H, Zhang FX, Liang JR. Drug-induced apoptosis in treatment of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:795-796. [DOI] |
| 23. | Gao H, Zhao ZH, Yang F. Treatment of GI tract cancer with arsenic troxidate to induce tumor cell apoptosis. Shijie Huaren Xiaohua Zazhi. 2002;10:710-711. [DOI] |
| 24. | Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, Tang W, Shi GY, Sun YP, Dai J. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772-778. [PubMed] [DOI] |
| 25. | Rousselot P, Labaume S, Marolleau JP, Larghero J, Noguera MH, Brouet JC, Fermand JP. Arsenic trioxide and melarsoprol induce apoptosis in plasma cell lines and in plasma cells from myeloma patients. Cancer Res. 1999;59:1041-1048. [PubMed] |
| 26. | Liu L, Qin S, Chen H, Wang J, Chen H, Ma J, Liu W. [An experimental study on arsenic trioxide-selectively induced human hepatocarcinoma cell lines apoptosis and its related genes]. Zhonghua Gan Zang Bing Za Zhi. 2000;8:367-369. [PubMed] |
| 27. | Zhang J, Xie DR, Yao HR, Lin XG, Liang XW, Xiang YQ. Arsenic trioxide induce apoptosis in colorectal cancer cell line SW620. Shijie Huaren Xiaohua Zazhi. 2001;9:228-229. [DOI] |
| 28. | Xu HY, Gao YY, Wu QL, Gao GQ, Yang YL, Chen SX, Liu TF. Proliferation inhibition and apoptosis induction by arsenic trioxide on human hepatoma cell line in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:1233-1237. [DOI] |
| 29. | Chen H, Qin S, Pan Q. [Antitumor effect of arsenic trioxide on mice experimental liver cancer]. Zhonghua Gan Zang Bing Za Zhi. 2000;8:27-29. [PubMed] |
| 30. | Chen HY, Liu WH, Qin SK. Induction of arsenic trioxide on apoptosis hepatocarcinoma cell lines. Shijie Huaren Xiaohua Zazhi. 2000;8:532-535. [DOI] |
| 31. | Yang YL, Xu HY, Gao YY, Wu QL, Gao GQ. Effects of arsenic trioxide on human hepatocarcinoma cell lines. Shijie Huaren Xiaohua Zazhi. 2000;8:1422-1423. [DOI] |
| 32. | Wang JM, Zou Q, Zou SQ. The role of bcl-2 gene in apoptosis of liver in rat with obstructive jaundice. Shijie Huaren Xiaohua Zazhi. 1999;7:1035-1037. [DOI] |
| 33. | Qiao Q, Wu JS, Zhang J, Ma QJ, Lai DN. Expression and significance of apoptosis related gene bcl-2 bax in human large intestine adenocarcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:936-938. [DOI] |
| 34. | Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15-17. [PubMed] [DOI] |
| 35. | Zhu ZH, Xia ZS, He SG. The effects of ATRA and 5-Fu on telomerase activity and cell growth of gastric cancer cells in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:669-673. [DOI] |
| 36. | Meng ZQ, Yu EX, Song MZ. Inhibition of telomerase activity of human liver cancer cell SMMC-7721 by chemotherapeutic drugs. Shijie Huaren Xiaohua Zazhi. 1999;7:252-254. [DOI] |
| 37. | Matthes E, Lehmann C. Telomerase protein rather than its RNA is the target of phosphorothioate-modified oligonucleotides. Nucleic Acids Res. 1999;27:1152-1158. [PubMed] [DOI] |
| 38. | Huang C, Ma WY, Li J, Dong Z. Arsenic induces apoptosis through a c-Jun NH2-terminal kinase-dependent, p53-independent pathway. Cancer Res. 1999;59:3053-3058. [PubMed] |
| 39. | Li YM, Broome JD. Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Res. 1999;59:776-780. [PubMed] |