修回日期: 2002-10-15
接受日期: 2002-10-22
在线出版日期: 2003-03-15
目的: 筛选获得HAb18G/CD147高亲和性拮抗肽中具有体外抗肝癌细胞转移作用的拮抗肽.
方法: 对于采用HAb18G/CD147纯抗原筛选噬菌体随机12肽库所获得的9条高亲和性的12肽(AP-1-AP-9). 分别采用: MTT法测定AP-1-AP-9的对肝癌细胞(HHCC)的直接毒性; 明胶酶谱法分析AP1-AP-9对基质金属蛋白酶(MMPs)产生及其激活过程的影响; 重组细胞基底膜(Matrigel)侵袭抑制实验测定AP-1-9对肝癌细胞(HHCC)侵袭力的的影响; 观察AP-1-AP-9对HHCC与细胞外基质蛋白(Matrigel, FN, LN, Collogen Ⅳ)、HHCC与间质细胞(成纤维细胞fb)黏附能力; 以及AP1-9对HHCC运动能力的影响.
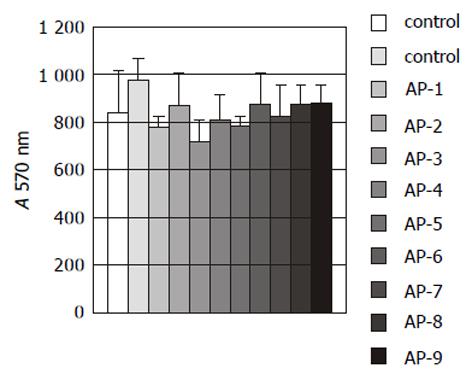
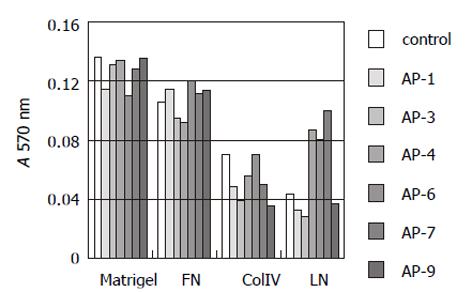
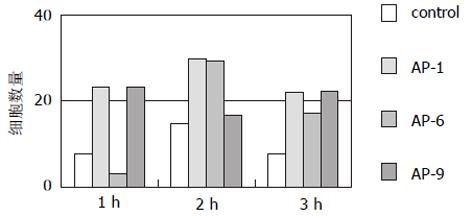
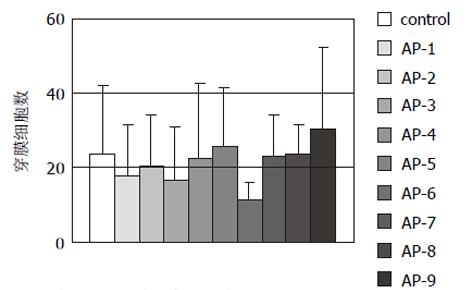
结果: Ⅳ拮抗肽AP-1-AP-9对HHCC无明显细胞毒性作用; AP-1, 6, 9对HHCC刺激fb细胞产生MMP-2其激活过程具有明显的抑制作用; AP-1, 3, 6, 7, 8, 9对HHCC侵袭力有明显的抑制作用(P<0.05), 抑制率分别为78.22%, 90.1%, 62.83%, 56.44%, 68.32%和81.19%; 9条拮抗肽对HHCC与细胞外基质蛋白Matrigel, FN无明显的抑制作用, 但AP-3, 9对HHCC与CollogenⅣ, LN的黏附有抑制作用; AP-1, 6, 9对HHCC与fb细胞之间的黏附有抑制作用; AP-6可抑制HHCC的运动能力, 抑制率54%, 但统计学上差异无显著性(P>0.05).
结论: HAb18G/CD147拮抗肽(AP-1-AP-9)对肝癌的侵袭转移相关的多个环节有明显的抑制作用, 为研究开发新的抗肿瘤转移多肽药物开辟了新途径.
引文著录: 骞爱荣, 商澎, 李郁, 黄宝成, 朱平, 梅其炳, 陈志南. HAb18G/CD147拮抗肽抗肝癌转移作用的体外实验. 世界华人消化杂志 2003; 11(3): 255-259
Revised: October 15, 2002
Accepted: October 22, 2002
Published online: March 15, 2003
AIM: To obtain the antagonistic peptides that have effect to prevent metastasis of Hepatocellular Carcinoma(HCC).
METHODS: Nine pieces of high affinity peptides (AP-1-AP-9) were obtained by pure HAb18G/CD147 antigen to panning phage displayed random peptide library. MTT assay was used to test toxicity of AP-1-AP-9 on human hepatocellular carcinoma cell (HHCC); Gelatin zymogram was used to analyze the effects of AP-1-AP-9 on activation and production of matrix metalloproteinase (MMPs); Matrigel-boyden chamber method was used to evaluate inhibitory abilities of AP-1-AP-9 on tumor cell invasion and metastasis; Inhibitory effect of AP-1-AP-9 on HHCC adhesion to extracellular matrix protein and fb cells were investigated. The influences of AP-1-AP-9 on HHCC chemotaxis migration were also tested.
RESULTS: AP-1-AP-9 has no toxicity on HHCC; AP-1, AP-6 and AP-9 could inhibit production and activation of MMP-2; the amounts of infiltrative cells in AP-1, 3, 6, 7, 8, 9 experimental groups are significantly less than that in control group (P < 0.05), and inhibition rate was 78.22%, 90.1%, 62.83%, 56.44%, 68.32%, 81.19%, respectively; AP-1-AP-9 had no effects on HHCC adhesion to matrigel and fibronectin (FN), whereas AP-3 and AP-9 could inhibit HHCC adhesion to collagen Ⅳand laminin(LN), AP-1, AP-6 and AP-9 could inhibit HHCC adhesion to fb. AP-6 could inhibit chemotaxis migration of HHCC with the inhibitory rate of 54% without statistical significance (P > 0.05).
CONCLUSION: HAb18G/CD147 antagonistic peptides (AP-1-AP-9) have inhibitory effects on many aspects or steps associated with metastasis of HCC, which provides avenue to explore medication for preventing metastasis of HCC.
- Citation: Qian AR, Shang P, Li Y, Huang BC, Zhu P, Mei QB, Chen ZN. Effects of HAb18G/CD147 antagonistic peptides on invasion and metastasis of HCC. Shijie Huaren Xiaohua Zazhi 2003; 11(3): 255-259
- URL: https://www.wjgnet.com/1009-3079/full/v11/i3/255.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i3.255
侵袭、转移行为是恶性肿瘤的本质特征. 肿瘤的转移规律极其复杂, 涉及诸多因素, 如蛋白水解酶; 血管生成因子; 黏附因子, 如整合素(integrin), 选择素(selectin), 钙黏合素(E-cadherin)等; 运动因子; 信号转导. 在肿瘤的侵袭、转移过程中, 癌细胞诱导效应细胞分泌蛋白水解酶, 降解细胞外基质, 其中基质金属蛋白酶(MMPs)最为重要. HAb18 是我室用肝癌抗原免疫小鼠得到的特异性单克隆抗体(mAb)[1], 我们用HAb18mAb从人肝癌组织cDNA文库中筛选得到其相应抗原HAb18G, 查询GENEBANK发现其基因序列与CD147分子序列一致, 并经鉴定HAb18 G与CD147为同一分子, 并在 此基础上进行了大量研究[2-6]. CD147分子, 又名EMMPRIN(细胞外基质金属蛋白酶诱导因子), 最初源于人肺癌细胞系LX-1[7,8], 在肝癌组织中发现尚属首次, 大量研究表明HAb18G/CD147与其效应细胞(癌周成纤维细胞)结合, 诱导产生基质金属蛋白酶(MMPs), 使肿瘤组织中MMPs含量及活性显著提高, 间质成分和血管基底膜的胶原蛋白过度降解, 癌细胞穿过基底膜及结缔组织屏障不断侵袭扩散, 低转移细胞经转染HAb18G/CD147后侵袭及转移能力增强[9,10]. 所以, 筛选有效的HAb18G/ CD147拮抗肽, 在高位阻断其引起效应细胞分泌MMPs, 是抗肿瘤转移的新途径. 为此, 我们采用HAb18G纯抗原筛选噬菌体随机12肽文库获得了9条与HAb18G/ CD147分子具有高度亲和力的多肽分子, 为获得具有抗肿瘤转移作用的拮抗肽采用细胞基底膜侵袭抑制实验、明胶酶谱、抗运动、抗黏附等实验进行亲和肽的功能筛选, 为进一步研究与开发肽类抗肿瘤转移药物提供新的备选化合物.
细胞系和试剂人肝癌细胞系 HHCC购自中科院上海细胞所细胞库; 人胚胎皮肤成纤维细胞(fb)西京医院烧伤科, 韩军涛博士馈赠; Millicell chamber购自Millipore 公司; Matrigel胶、层粘连蛋白(LN)购自BD公司; 纤维粘连蛋白(FN)、 Ⅳ型胶原(Col Ⅳ)、 DMEM培养基购自GIBCO公司; 四氮唑(MTT)、二甲基亚砜(DMSO)购自华美公司. HAb18G/ CD147拮抗肽(APs)采用固相合成法合成9条HAb18G/ CD147拮抗肽(AP1-9), 成品经高效液色谱分析>90%, MALDI-TOF质谱分析分子质量符合理论值. Heraeus CO2二氧化碳培养箱(德国Heraeus公司), 酶标仪(Bio-Rad), 图像分析仪(上海复日科技有限公司), 96, 24孔板(Nunc, 瑞典).
1.2.1 细胞毒性实验: 人肝癌细胞(HHCC)培养至对数期, 用2.5 g/L 胰酶消化, 以8×104/孔200 μL的密度接种于96孔板中同时加入20 μL (1 g/L)APs, 37℃孵育2 h; 弃取培养液, 加入PBS200 mL/孔, 吹打3遍, 加入MTT 40 μg/孔, 37℃孵育4 h. 弃取MTT, 加入200 μL DMSO, 酶标仪570 nm测定吸光度A值.
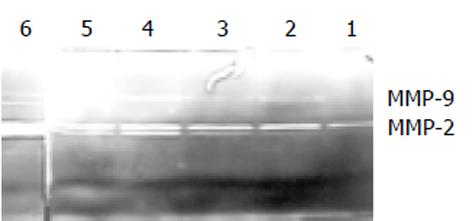
1.2.2 改良的明胶酶谱法[7]: 人成纤维细胞(Fb)和人肝癌细胞(HHCC)培养至对数期, 用2.5 g/L 胰酶消化, 以105/孔的密度接种于96孔板中(Fb细胞和HHCC细胞的体积比为1: 1, 共200 m L), 完全DMEM培养24 h. 弃去培养上清, 用PBS洗2次后, 换无血清DMEM培养液培养1 h, 再用PBS洗2次, 加300 μ L无血清DMEM培养液共培养20 h. 在培养后的5, 10, 15和20h收集无血清DMEM培养细胞上清, 低速离心(200 g)去除细胞碎片后, 4℃备用. 上清进行SDS-PAGE电泳, 其中分离胶含1 g·L-1的明胶. 电泳结束后将凝胶置于洗脱液(25 mg·L-1 Triton X-100, 50 mmol·L-1 Tris-HCl, pH7.5, 0.1mol·L-1 NaCl)室温洗2次, 45min/次, 接着将凝胶置于孵育液(50 mmol·L-1 Tris-HCl, PH7.5, 10 mmol·L-1 CaCl2, 0.2 g·L-1 NaN3)37℃孵育24 h以上, 考马斯亮蓝染色, 脱色液脱色, 蓝色背景上出现透亮带. 经图像分析系统读取条带面积和酶解条带灰度, 酶含量 = 条带面积×(条带灰度-背景灰度).
1.2.3 重组基底膜侵袭抑制实验: 采用Matrigel-Boyden方法观察Millicell小室的滤膜保证其正常, 将小室放入灭菌的24孔板中; 在Millicell小室内膜上加入Matrigel胶(40 mL/膜), 37℃ 1 h, 超净台内吹干; 在小室的外部24孔板内加入趋化剂200 μL; 用2.5 g/L胰酶消化HHCC和fb细胞, 离心洗涤3次, DMEM(含1 g/L BCS) 悬起, 1: 1(各150 uL)加入上室, 同时在上室加入拮抗肽20 ml(1 mg/mL), 在下室加入200 μL不完全DMEM(含0.1%BCS), 37℃, 50 mL/L CO2培养20 h; 取出小室, 弃去培养液, 用棉签擦去上室中的细胞, 950 mL/L酒精固定15 min后, 取下滤膜; 固定后的滤膜HE染色, 中性树胶封片, 在40×光镜下计数膜背面侵袭的细胞数, 随机计数中间和四周共5个视野, 每细胞计数3份, 求出其平均值, 测验重复3次.
1.2.4 拮抗肽抗HHCC与细胞外基质黏附实验: 96孔板中分别铺上LN或FN或Matrigel各2 μg, 室温干燥; 加入20 g/L BSA 20 μL/孔, 37℃孵育1 h, PBS冲洗; 以含1 g/L BCS的DMEM 悬浮HHCC细胞, 每孔加入HHCC细胞200 μL及10 μL (1 g/L)拮抗肽, 37℃孵育2 h; 弃取培养液, 加入PBS 200 μL/孔, 吹打3遍, 加入MTT 40 μg/孔, 37℃孵育4 h; 弃取MTT, 加入DMSO 200 μL, 酶标仪570 nm测定吸光度A值.
1.2.5 拮抗肽抗HHCC与人fb黏附实验: 用2.5 g/L 胰酶消化生长至对数期的人成纤维细胞(fb), 离心, 不完全DMEM 悬浮fb细胞(1×105), 96孔板中, 每孔加入200 μL, 37℃, 50 mL/L CO2培养24 h. 弃去96孔板中的培养液, PBS洗二次, 加入用2.5 g/L 胰酶消化后, 不完全DMEM 悬浮的HHCC细胞(1×105), 同时加入拮抗肽(10-80 mg). 分别在1, 2, 3 h各自复孔中, 分别取出上清, 并用PBS冲洗3次, 合并提取液, 离心, 用PBS100 mL悬浮沉淀, 血球计数板计数, 每样重复计数3次, 取平均值.
1.2.6 抗趋化运动实验: 在小室下室面涂趋化剂FN10 mg, 室温干燥; 上室加入2×105 HHCC细胞300 mL, 同时加入(1g/L)拮抗肽20 μL, 下室加入1 g/L BCS的DMEM300 mL. 37℃, 50mL/L CO2培养20 h; 取出小室, 弃去培养液, 用棉签擦去上室中的细胞, 950 mL/L酒精固定15 min后, 取下滤膜; 固定后的滤膜HE染色, 中性树胶封片, 在40×光镜下计数膜背面穿过的细胞数, 随机计数中间和四周共5个视野, 每细胞计数3份, 求出其平均值, 测验重复2次.
统计学处理 单因素方差分析.
5-15 h动态明胶酶谱法中, AP-9对MMP-2和MMP-9的产生和激活有明显的抑制作用(图2). 图2是在不同时间点采样, 进行的酶谱分析. 测定了给予AP-9后5, 10, 15 h时间段内明胶酶的变化. 其中1, 3, 5条带分别为给药组, 2, 4, 6条带为对照组. 从该图可发现给药组各时间点明胶酶的含量均低于对照组各时间点, 随着时间的延长, 明胶酶的量也在逐渐增加; 另外随着时间的延长酶原形式的ProMMP-2, 9以及活性形式的MMP-2, 9产生均增加, 15 h达到最高
HHCC单独培养组与HHCC+fb共培养组相比较, 穿膜细胞数明显少于后者(P<0.05), HHCC和fb共培养并加入APs的9个实验组的AP-1, 3, 6, 7, 8, 9六条肽穿膜细胞数明显少于对照组(P<0.05 表1).
APs中的AP-1, 3, 4, 6, 7, 9对HHCC与Matrigel, FN黏附无明显的抑制作用, 但AP-3, 9能抑制HHCC与 CollogenⅣ, LN的黏附作用(图3).
AP-1, AP-9在1, 2, 3 h, AP-6在2, 3h能明显抑制HHCC细胞与fb细胞之间的黏附(图4).
CD147分子是广泛表达于造血及非造血细胞系, 分子质量为50-60 ku的跨膜糖蛋白, 属免疫球蛋白超家族(IgSF)成员. 编码CD147的基因定位于人19号染色体19p13.3[7], 人CD147分子曾有多种不同的命名, 包括TCSF/EMMPrin[13,14], M6[11], Basigin[12], Neurothelin[15,16]. 如此多的命名也反映其在不同的组织中有不同的功能. 我们曾经用表达HAb18G/CD147的CHO细胞刺激人成纤维细胞, 证实成纤维细胞分泌MMPs的量大大增加, 即其具有EMMPRIN的功能. EMMPRIN, 能够结合于内皮细胞及纤维母细胞[17], 因纯化的该蛋白可刺激人成纤维细胞分泌基质金属蛋白酶(MMPs)而得名. 恶性肿瘤的转移虽然极其复杂, 但在其侵袭、转移演进过程中, 癌细胞必须首先具备降解细胞外结缔组织间质和血管基底膜这些天然屏障的能力, 这依赖于蛋白水解酶的作用. 蛋白水解酶主要包括四类, 即: 丝氨酸蛋白酶(如纤溶酶原激活物PAs)、半胱氨酸蛋白酶(如组织蛋白酶B)以及天门冬氨酸蛋白酶(如胃蛋白酶)和基质金属蛋白酶MMPs. 其中基质金属蛋白酶最为重要, 大量研究已表明, 恶性肿瘤组织中MMPs的含量及活性显著高于其周围正常组织, 且与肿瘤恶性表型密切正相关[18], 因而是肿瘤具备高转移潜能的一个客观、良好的分子指标, 已逐渐成为近年来肿瘤侵袭、转移研究的热点. MMPs在肿瘤转移中的作用可概括为: 破坏局部组织结构, 促进肿瘤生长; 破坏基底膜屏障, 利于肿瘤转移; 通过对细胞外基质的改建, 促进肿瘤新生血管的形成. 近来的文献报道, CD147分子不仅能刺激MMPs的产生, 而且还与MMPs在肿瘤细胞表面形成复合物, 这种复合物的形成相当于将MMPs浓缩在肿瘤细胞周围, 有利于肿瘤细胞周围基质的降解, 使肿瘤细胞易于扩散和转移[19]. 目前, 抗间质降解多采用酶抑制剂, 如天然酶抑制剂-组织型金属蛋白酶抑制剂(TIMPs), 能够与MMPs结合并抑制酶活性, 但TIMPs在组织中含量极微且难于提取, 人工重组TIMPs是大分子蛋白质, 存在降解和口服吸收不良等问题. 人工合成的拟肽类金属蛋白酶抑制剂, 如Batimastat (BB-94)由于其给药方式的局限和生物利用度低等原因, 导致Ⅲ期临床实验的停止[20]. 目前MMPs的抑制剂(MMPIs)Marimastat及其类似物现已进入临床Ⅱ-Ⅳ期, 均能不同程度的改善患者的生活质量[21].
目前关于肽类抗肿瘤转移抑制剂的研究主要有以下几种: RGD(Arg-Gly-Asp)三肽, 他通过结合和饱和细胞表面的整合素、纤维粘连蛋白(FN)和层粘连蛋白(LN)结合位点来抑制肿瘤的黏附、浸润和转移[22-25]; RGDS (Arg-Gly-Asp-Ser)四肽的类似物抑制肿瘤细胞黏附到FN、玻璃粘连蛋白(vitronectin, VN)和RGDS底物, 从而抑制肿瘤转移[26-28]; YIGSR, 是一合成 的LN的5肽, 可以抑制肿瘤细胞与内皮细胞的黏附及肿瘤细胞的侵袭能力[29-31]; 来自col IV胶原的合成肽有二种, 一种是来自Col IV胶原α1链胶原结构域的15肽(IV-H1), 可以促进肿瘤细胞的浸润、黏附和转移, 但是抗IV-H1肽抗体可抑制肿瘤细胞的黏附、运动和转移[32]. 另一种是来自Col IV胶原α3链非胶原结构域(185-203), 可以抑制黑素瘤细胞和成纤维肉瘤细胞的运动、膜结合的MMP-2的活化、激活设计到黏附斑激酶(FAK)和PI3激酶的信号转导通路[33,34]; 来自FN的合成肽通过抑制肿瘤细胞的运动能力、整合素介导的黏附、VN刺激的酪氨酸磷酸酶包括p125(FAK)和纽带蛋白以及整合素介导的信号转导等多个环节, 从而抑制肿瘤的转移[35,36]; 其他的一些肽类抑制剂包括一些丝氨酸蛋白酶、基质金属蛋白酶抑制剂等均可通过抑制肿瘤转移相关的几个环节来抑制肿瘤的转移[37-40].
在此基础上, 我们采用HAb18G/CD147纯化抗原筛选噬菌体随机12肽文库, 得到了9条与抗原高度亲和的12肽, 再通过体内外抗转移活性功能性筛选, 欲获得可以在高位阻断HAb18G/CD147诱导效应细胞分泌MMPs, 以期获得对肿瘤复发转移的治疗拮抗肽. 我们筛选获得的拮抗肽AP-1-9荷瘤裸鼠体内实验结果表明: APs具有抑制皮下移植瘤生长, 防止肝内移植瘤播散、转移和延长荷瘤裸鼠生存期的作用. APs还对鸡胚绒毛尿囊膜(CAM)和Matrigel plug 小鼠体内血管生成具有抑制作用. 经生物信息学分析与黏附、凋亡、血管生成及信号转导相关蛋白高度同源. 经激光共聚焦和流式细胞仪证实HAb18G/CD147拮抗肽与肝癌细胞表面抗原HAb18G/CD147有较高的亲和性. 我们经体外实验证明, 对肝癌细胞转移的抑制率可高达90%. 通过细胞毒性实验结果证明各组与对照组之间差别无显著性, 这提示该拮抗肽无直接的细胞毒性, 其作用机制不是直接杀伤或杀死癌细胞, 进一步证实本实验的原理是阻断MMPs的产生. 此外, 本实验中拮抗肽对肿瘤细胞与细胞外基质之间的黏附无拮抗作用, 而对肿瘤细胞与间质细胞之间的黏附有拮抗作用, 抗运动实验在统计学上差别无显著性, 该结果也进一步证实我们设计的靶点是正确的, 是通过特异的阻断MMPs产生, 进而抑制新生血管的形成, 从而抑制肿瘤的侵袭、转移, 提高生命延长率.
总之, HAb18G/CD147拮抗肽是从高位阻断效应细胞分泌MMPs, 从而抑止肿瘤的浸润和转移, 为肿瘤转移的治疗提供了新的途径.
编辑: N/A
| 1. | Chen ZN. [Radiolocalization of human hepatoma with anti-human hepatoma monoclonal antibodies and its F(ab')2 in tumor-bearing nude mice]. Zhonghua Yi Xue Za Zhi. 1989;69:566-568, 38. [PubMed] |
| 2. | Chen ZN. [Significance and application of anti-malignant hepatoma MAb HAb18 in radioimmunal diagnosis of human hepatocellular carcinoma]. Zhonghua Zhong Liu Za Zhi. 1992;14:9-12. [PubMed] |
| 3. | Lou C, Chen ZN, Bian HJ, Li J, Zhou SB. Pharmacokinetics of radioimmunotherapeutic agent of direct labeling mAb 188Re-HAb18. World J Gastroenterol. 2002;8:69-73. [PubMed] [DOI] |
| 4. | Yang LJ, Sui YF, Chen ZN. Preparation and activity of conjugate of monoclonal antibody HAb18 against hepatoma F(ab')(2) fragment and staphylococcal enterotoxin A. World J Gastroenterol. 2001;7:216-221. [PubMed] [DOI] |
| 5. | Bian HJ, Chen ZN, Deng JL. Direct technetium-99m labeling of anti-hepatoma monoclonal antibody fragment:a radioimmunoconjugate for hepatocellular carcinoma imaging. World J Gastroenterol. 2000;6:348-352. [PubMed] [DOI] |
| 6. | Qiu K, Wang BC, Chen ZN, Fang P, Liu CG, Wan WX, Liu YF. 99mTc-labeled HAb18 McAb Fab fragment for radioimmunoimaging in nude mice bearing human hepatocellular carcinoma. World J Gastroenterol. 1998;4:117-120. [PubMed] [DOI] |
| 7. | Ellis SM, Nabeshima K, Biswas C. Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res. 1989;49:3385-3391. [PubMed] |
| 8. | Nabeshima K, Lane WS, Biswas C. Partial sequencing and characterization of the tumor cell-derived collagenase stimulatory factor. Arch Biochem Biophys. 1991;285:90-96. [PubMed] [DOI] |
| 9. | Jiang JL, Zhou Q, Yu MK, Ho LS, Chen ZN, Chan HC. The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J Biol Chem. 2001;276:46870-46877. [PubMed] [DOI] |
| 10. | Leber TM, Balkwill FR. Zymography: a single-step staining method for quantitation of proteolytic activity on substrate gels. Anal Biochem. 1997;249:24-28. [PubMed] [DOI] |
| 11. | Kasinrerk W, Fiebiger E, Stefanová I, Baumruker T, Knapp W, Stockinger H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol. 1992;149:847-854. [PubMed] |
| 12. | Kaname T, Miyauchi T, Kuwano A, Matsuda Y, Muramatsu T, Kajii T. Mapping basigin (BSG), a member of the immunoglobulin superfamily, to 19p13.3. Cytogenet Cell Genet. 1993;64:195-197. [PubMed] [DOI] |
| 13. | Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434-439. [PubMed] |
| 14. | Guo H, Zucker S, Gordon MK, Toole BP, Biswas C. Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem. 1997;272:24-27. [PubMed] [DOI] |
| 15. | Seulberger H, Unger CM, Risau W. HT7, Neurothelin, Basigin, gp42 and OX-47--many names for one developmentally regulated immuno-globulin-like surface glycoprotein on blood-brain barrier endothelium, epithelial tissue barriers and neurons. Neurosci Lett. 1992;140:93-97. [PubMed] [DOI] |
| 16. | Schlosshauer B, Herzog KH. Neurothelin: an inducible cell surface glycoprotein of blood-brain barrier-specific endothelial cells and distinct neurons. J Cell Biol. 1990;110:1261-1274. [PubMed] [DOI] |
| 17. | Kasinrerk W, Tokrasinwit N, Phunpae P. CD147 monoclonal antibodies induce homotypic cell aggregation of monocytic cell line U937 via LFA-1/ICAM-1 pathway. Immunology. 1999;96:184-192. [PubMed] [DOI] |
| 18. | Davies B, Miles DW, Happerfield LC, Naylor MS, Bobrow LG, Rubens RD, Balkwill FR. Activity of type IV collagenases in benign and malignant breast disease. Br J Cancer. 1993;67:1126-1131. [PubMed] [DOI] |
| 19. | Guo H, Li R, Zucker S, Toole BP. EMMPRIN (CD147), an inducer of matrix metalloproteinase synthesis, also binds interstitial collagenase to the tumor cell surface. Cancer Res. 2000;60:888-891. [PubMed] |
| 20. | Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642-6650. [PubMed] [DOI] |
| 21. | Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387-2392. [PubMed] [DOI] |
| 22. | Tsuchiya Y, Sawada S, Tsukada K, Saiki I. A new pseudo-peptide of Arg-Gly-Asp (RGD) inhibits intrahepatic metastasis of orthotopically implanted murine hepatocellular carcinoma. Int J Oncol. 2002;20:319-324. [PubMed] |
| 23. | Pasqualini R, Koivunen E, Ruoslahti E. A peptide isolated from phage display libraries is a structural and functional mimic of an RGD-binding site on integrins. J Cell Biol. 1995;130:1189-1196. [PubMed] [DOI] |
| 24. | Sogabe T, Nishimura S, Chung YS, Sowa M. [Role of tripeptide Arg-Gly-Asp (RGD) on adhesiveness of human pancreatic cancer cell, PANC-1 to extracellular matrix]. Nihon Rinsho. 1995;53:1648-1652. [PubMed] |
| 25. | Gehlsen KR, Argraves WS, Pierschbacher MD, Ruoslahti E. Inhibition of in vitro tumor cell invasion by Arg-Gly-Asp-containing synthetic peptides. J Cell Biol. 1988;106:925-930. [PubMed] [DOI] |
| 26. | Komazawa H, Saiki I, Aoki M, Kitaguchi H, Satoh H, Kojima M, Ono M, Itoh I, Azuma I. Synthetic Arg-Gly-Asp-Ser analogues of the cell recognition site of fibronectin that retain antimetastatic and anti-cell adhesive properties. Biol Pharm Bull. 1993;16:997-1003. [PubMed] [DOI] |
| 27. | Saiki I, Koike C, Obata A, Fujii H, Murata J, Kiso M, Hasegawa A, Komazawa H, Tsukada H, Azuma I. Functional role of sialyl Lewis X and fibronectin-derived RGDS peptide analogue on tumor-cell arrest in lungs followed by extravasation. Int J Cancer. 1996;65:833-839. [PubMed] [DOI] |
| 28. | Fujii H, Komazawa H, Mori H, Kojima M, Itoh I, Murata J, Azuma I, Saiki I. Antimetastatic activities of synthetic Arg-Gly-Asp-Ser (RGDS) and Arg-Leu-Asp-Ser (RLDS) peptide analogues and their inhibitory mechanisms. Biol Pharm Bull. 1995;18:1681-1688. [PubMed] [DOI] |
| 29. | Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, Martin GR. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132-1134. [PubMed] [DOI] |
| 30. | Iwamoto Y, Fujita Y, Sugioka Y. YIGSR, a synthetic laminin peptide, inhibits the enhancement by cyclophosphamide of experimental lung metastasis of human fibrosarcoma cells. Clin Exp Metastasis. 1992;10:183-189. [PubMed] [DOI] |
| 31. | Koliakos G, Trontzos C, Kouzi-Koliakos K, Kanellaki M, Grammaticos P. Lung carcinoma imaging using a synthetic laminin derivative radioiodinated peptide YIGSR. J Nucl Med. 1997;38:1940-1944. [PubMed] |
| 32. | Chelberg MK, McCarthy JB, Skubitz AP, Furcht LT, Tsilibary EC. Characterization of a synthetic peptide from type IV collagen that promotes melanoma cell adhesion, spreading, and motility. J Cell Biol. 1990;111:261-270. [PubMed] [DOI] |
| 33. | Pasco S, Han J, Gillery P, Bellon G, Maquart FX, Borel JP, Kefalides NA, Monboisse JC. A specific sequence of the noncollagenous domain of the alpha3(IV) chain of type IV collagen inhibits expression and activation of matrix metalloproteinases by tumor cells. Cancer Res. 2000;60:467-473. [PubMed] |
| 34. | Pasco S, Monboisse JC, Kieffer N. The alpha 3(IV)185-206 peptide from noncollagenous domain 1 of type IV collagen interacts with a novel binding site on the beta 3 subunit of integrin alpha Vbeta 3 and stimulates focal adhesion kinase and phosphatidylinositol 3-kinase phosphorylation. J Biol Chem. 2000;275:32999-33007. [PubMed] [DOI] |
| 35. | Humphries MJ, Olden K, Yamada KM. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986;233:467-470. [PubMed] [DOI] |
| 36. | Kato R, Ishikawa T, Kamiya S, Oguma F, Ueki M, Goto S, Nakamura H, Katayama T, Fukai F. A new type of antimetastatic peptide derived from fibronectin. Clin Cancer Res. 2002;8:2455-2462. [PubMed] |
| 37. | Kanemoto T, Martin GR, Hamilton TC, Fridman R. Effects of synthetic peptides and protease inhibitors on the interaction of a human ovarian cancer cell line (NIH:OVCAR-3) with a reconstituted basement membrane (Matrigel). Invasion Metastasis. 1991;11:84-92. [PubMed] |
| 38. | Melchiori A, Albini A, Ray JM, Stetler-Stevenson WG. Inhibition of tumor cell invasion by a highly conserved peptide sequence from the matrix metalloproteinase enzyme prosegment. Cancer Res. 1992;52:2353-2356. [PubMed] |
| 39. | Yamada KM, Kennedy DW, Yamada SS, Gralnick H, Chen WT, Akiyama SK. Monoclonal antibody and synthetic peptide inhibitors of human tumor cell migration. Cancer Res. 1990;50:4485-4496. [PubMed] |
| 40. | DeRoock IB, Pennington ME, Sroka TC, Lam KS, Bowden GT, Bair EL, Cress AE. Synthetic peptides inhibit adhesion of human tumor cells to extracellular matrix proteins. Cancer Res. 2001;61:3308-3313. [PubMed] |