修回日期: 2002-08-20
接受日期: 2002-09-03
在线出版日期: 2003-02-15
目的: 观察大鼠实验性溃疡性结肠炎肠组织核因子-κB(NF-κB)及TNF-α、ICAM-1的表达, 探讨NF-κB在溃疡性结肠炎发病过程中的作用.
方法: 采用三硝基苯磺酸(TNBS)制备大鼠溃疡性结肠炎模型, 设正常组、生理盐水组及轻、重模型组共4组. 采用免疫组织化学染色检测肠组织NF-κB、TNF-α和ICAM-1表达, 生化方法检测MPO含量, 并分析NF-κB活性与肠道病理损伤指数、MPO含量、TNF-α及ICAM-1表达的关系.
结果: 溃疡性结肠炎大鼠肠组织中NF-κB活性, TNF-α、ICAM-1表达及MPO含量较正常组及生理盐水组显著增高(NF-κB: 52.14±9.81、30.26±10.20, 60.73±13.41、45.24±10.86 vs 13.31±4.76、16.95±6.83, 11.61±4.85、14.10±5.76; TNF-α: 74.50±11.20、48.11±5.95, 84.09±14.52、53.40±8.79 vs 16.99±5.48、20.04±6.76, 10.13±1.79、16.03±6.21; ICAM-1: 68.15±7.25、44.34±7.54, 77.69±8.09、47.01±8.82 vs 15.34±4.03、17.50±6.95, 10.33±2.38、13.41±4.91; MPO : 1.69±0.11, 0.71±0.06 vs 0.39±0.07, 0.31±0.08; P<0.01), 且在病情严重组增高尤为明显. NF-κB表达水平与TNF-α和ICAM-1阳性细胞密度、MPO含量、肠道病理损伤指数呈显著正相关关系(r分别为0.9 304, 0.8 680, 0.6 865, 0.9 292, 0.8 462; P<0.001或P<0.005).
结论: NF-κB在溃疡性结肠炎发病过程中可能起重要作用.
引文著录: 李军华, 于皆平, 何小飞, 徐细明. 核因子-κB在大鼠实验性溃疡性结肠炎肠组织的表达及其意义. 世界华人消化杂志 2003; 11(2): 214-218
Revised: August 20, 2002
Accepted: September 3, 2002
Published online: February 15, 2003
AIM: To investigate the role of NF-kB in the pathogenesis of 2, 4, 6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats.
METHODS: Ulcerative colitis model in rat was induced by TNBS. The expression of NF-kB, TNF-α and ICAM-1 protein in colon tissues were detected by immunohistochemistry. The myeloperoxidase (MPO) activity in colon mucosa was detected by biochemistry. The pathological changes in the colon were examined, the relationships among the activity of NF-κB, TNF-α, ICAM-1, and MPO were evaluated.
RESULTS: The activity of NF-kB and the expression of TNF-α, ICAM-1 in rats with TNBS-induced colitis was higher than that in normal control (P < 0.01), a very significant correlation between the expression of NF-kB: 52.14±9.81±30.26±10.20, 60.73±13.41, 45.24±10.86 vs 13.31±4.76, 16.95±6.83, 11.61±4.85, 14.10±5.76; with TNF-α, 74.50±11.20, 48.11±5.95, 84.09±14.52, 53.40±8.79 vs 16.99±5.48, 20.04±6.76, 10.13±1.79, 16.03±6.21; ICAM-1: 68.15±7.25, 44.34±7.54, 77.69±8.09, 47.01±8.82 vs 15.34±4.03, 17.50±6.95, 10.33±2.38, 13.41±4.91, MPO: 1.69±0.11, 0.71±0.06 vs 0.39±0.07, 0.31±0.08; ICAM-1, MPO activity, macroscopic and histological changes in TNBS-induced colitis was found, respectively (r = 0.9304, 0.8680, 0.6865, 0.9 292, 0.8 462; P < 0.001 or P < 0.005).
CONCLUSION: NF-κB may play an important role in the pathogenesis of ulcerative colitis in rats.
- Citation: Li JH, Yu JP, He XF, Xu XM. Expression of NF-kB in rats with TNBS-induced ulcerative colitis. Shijie Huaren Xiaohua Zazhi 2003; 11(2): 214-218
- URL: https://www.wjgnet.com/1009-3079/full/v11/i2/214.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i2.214
溃疡性结肠炎(UC)为慢性非特异性的肠道炎症性疾病, 近年来, 国内报道逐渐增多[1], 但其病因、发病机制尚未完全明确[2-4]. -般认为, 免疫异常是其发病的主要原因[5-7]. 近年研究表明细胞因子, 黏附分子及其他炎性递质表达异常与UC免疫病理机制关系密切[8-16]. 核因子-κB(nuclear factor-κB, NF-κB)是-具有多向性转录调节作用的核蛋白因子, 广泛参与多种基因特别是免疫炎症反应相关基因的表达调控[17-23], 在机体炎症反应过程中发挥重要作用. 目前有关NF-κB与溃疡性结肠炎关系的研究尚未见报道. 本研究通过观察大鼠实验性溃疡性结肠炎肠组织中NF-κB活性变化及其与肠组织病理改变, 炎性损伤之间的关系, 探讨NF-κB与溃疡性结肠炎发病关系及在其发生发展中的作用, 进一步阐明溃疡性结肠炎的发病机制, 为临床治疗开辟新的途径.
(1)动物: SPF级健康成年SD大鼠32只, 雌雄不拘, 体重150-200 g, 正常喂养. 由湖北省医学科学院实验动物中心提供. (2)主要试剂: 2、4、6-三硝基苯磺酸(TNBS)(Sigma USA); NF-κB P65、TNF-α和ICAM-1单克隆抗体 (santa cruz biotechnology公司); SP试剂盒为北京中山公司产品; MPO检测试剂盒为南京建成生物工程研究所产品; 其他试剂为进口或国产分析纯.
32只SD大鼠随机分为正常组、生理盐水组、模型Ⅰ、Ⅱ组共4组, 正常组不作任何处理, 生理盐水组(NS组)以0.9%生理盐水溶液代替三硝基苯磺酸(TNBS)作阴性对照. 模型组以TNBS灌肠制备溃疡性结肠炎模型. 大鼠溃疡性结肠炎模型制备, 实验大鼠术前禁食24 h, 自由饮水. 实验时乙醚麻醉后, 用-直径2.0 mm, 长约12 cm的硅胶管由肛门轻缓插入深约8 cm, 生理盐水组以1 mL/只的0.9%生理盐水溶液, 模型Ⅰ、Ⅱ组分别以含50 mg/kg、150 mg/kg TNBS的50%乙醇溶液缓缓推入结肠, 诱导轻重不同的溃疡性结肠炎形成. 动物保持平躺自然清醒. 模型建立3 wk后处死动物, 取肛门至盲肠段结肠(约8 cm), 沿肠系膜纵轴剪开, 冷生理盐水冲洗干净, 肉眼进行大体形态评分. 肠组织-部分以4.0%多聚甲醛固定, 石蜡包埋, 4 um连续切片, HE染色镜下评价炎症和溃疡, 另一部分-20℃冻存, 用以MPO检测. 肠组织NF-κB、TNF-α和ICAM-1的表达, 采用免疫组织化学SP法, 取石蜡切片(4 um)常规脱蜡、水化, 置0.05 M柠檬酸缓冲液中进行微波抗原修复, 凉至室温; 3% H2O2孵育10 min以消除内源性过氧化物酶活性, 滴加10%正常血清封闭非特异性抗原; 倾去血清, 滴加1: 100稀释的NF-κB、TNF-α和ICAM-1抗体; 4℃放置过夜, PBS冲洗3×5 min, 滴加生物素化二抗, 37℃孵育10 min, PBS冲洗3×5 min; 滴加辣根过氧化物酶标记的卵白素工作液, 37℃孵育15 min; DAB显色, 苏木素复染, 中性树脂封片. 阳性对照为已知的TNF-α和ICAM-1蛋白, 以PBS代替-抗作阴性对照. 每张切片随机选取5个高倍视野, 计数1 000个细胞, 计算阳性细胞百分比; 以16 D目镜测微网(面积为0.1 024 mm2)在400倍放大下计数阳性细胞数, 每张切片计算10个网格取平均值为阳性细胞密度. 结肠组织MPO含量测定, 采用生化方法, 具体操作按试剂盒说明书进行. 结肠组织损伤大体形态和组织学形态评分方法[24,25]: 大体形态损伤评分指标包括粘连、局部充血、溃疡及炎症. 粘连及充血按有无及轻重分别计0、1、2分, 出现炎症、溃疡数目增加1个、溃疡面积>2 cm时范围每增加1 cm计分均加1.组织学指标包括溃疡、炎症、肉芽肿、纤维化及病变深度, 按有无及轻重分别计0、1、2分, 病变深度达黏膜下层、肌层、浆膜层分别计1、2、3分, 各项相加得总分.
统计学处理 实验数据以表示, 采用单因素方差分析, 组间差异采用q检验, 相关关系分析采用直线相关分析. 以P<0.01为有统计学意义.
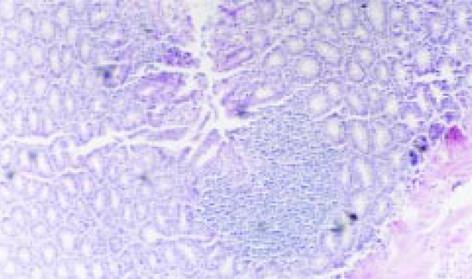
模型组大鼠结肠标本表现为黏膜充血水肿、溃疡形成, 结肠组织与周围组织粘连, 可见近端肠腔扩张. 溃疡呈线状和灶状, 周围可见黏膜增厚, 肠腔狭窄. 炎症及溃疡位于盲肠部与肛门之间. 光镜下可见溃疡及炎性渗出物, 有淋巴组织增生, 大量中性粒细胞浸润, 可见数量不等的单个核细胞和嗜酸性粒细胞, 同时可见增生的纤维母细胞; 与模型Ⅰ组相比, 模型Ⅱ组大鼠肠组织损伤的大体形态及组织学评分明显增高(P<0.01), 见图1, 表1.
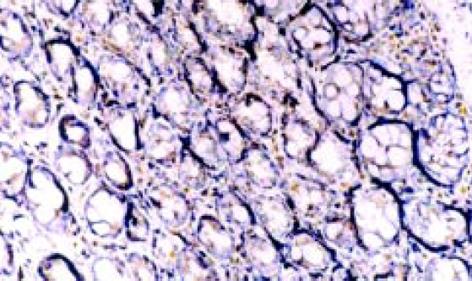
NF-κ B阳性表达细胞胞核和胞质都有着色, 以胞核为主, 阳性着色细胞主要为黏膜固有层单核细胞, 肠上皮细胞也有少量着色. 正常组及生理盐水组仅见微弱的NF-κ B表达(14.10±5.76, 16.95±6.38), 模型Ⅰ、Ⅱ组NF-κ B表达水平明显增高(45.24±10.86, 60.73±13.41 P<0.01), 尤其以模型Ⅱ组更为明显(P<0.01), 见表2、图2.
| 分组 | NF-κB | TNF-α | ICAM-1 | |||
| 阳性率(%) | 阳性密度 | 阳性率(%) | 阳性密度 | 阳性率(%) | 阳性密度 | |
| 正常组 | 11.61±4.85 | 14.10±5.76 | 10.13±1.79 | 16.03±6.21 | 10.33±2.38 | 13.41±4.91 |
| NS组 | 13.31±4.76 | 16.95±6.83 | 16.99±5.48 | 20.04±6.76 | 15.34±4.03 | 17.50±6.95 |
| 模型Ⅰ组 | 30.26±10.20bd | 45.24±10.86bd | 48.11±5.95bd | 53.40±8.79bd | 44.34±7.54bd | 47.01±8.82bd |
| 模型Ⅱ组 | 52.14±9.81bdf | 60.73±13.41bdf | 74.50±11.20bdf | 84.09±14.52bdf | 68.15±7.25bdf | 77.69±8.09bdf |
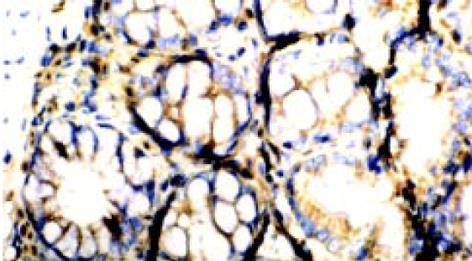
TNF-α和ICAM-1阳性表达细胞表现以胞质呈棕褐色为主, 胞核也有少量着色. 阳性表达细胞有中性粒细胞、单个核细胞等, 多集中于靠近肌层的黏膜固有层内, 肠上皮细胞仅有少量阳性表达, 黏膜内小血管内皮细胞也可见ICAM-1阳性表达. 见图3、4.正常组及生理盐水组肠组织仅见极少量的TNF-α及ICAM-1蛋白表达, 模型组有不同程度的阳性染色, 阳性率和阳性细胞密度明显高于正常组及生理盐水组(P<0.01), 模型组中Ⅱ组阳性表达明显高于Ⅰ组(P<0.01), 见表2.
模型组与正常组及生理盐水组相比, 肠组织MPO含量显著上升(0.71±0.06, 1.69±0.11 vs 0.31±0.08, 0.39±0.07, P<0.01), 模型组Ⅱ组则明显高于模型Ⅰ组(P<0.01), 见表1.
溃疡性结肠炎大鼠肠组织NF-κB阳性细胞密度与肠组织损伤大体形态及组织学评分及MPO含量呈显著正相关关系(r分别为0.9 292, 0.8 462, 0.6 865, P<0.005).
溃疡性结肠炎大鼠肠组织NF-κB阳性细胞密度与TNF-α和ICAM-1蛋白阳性细胞密度呈显著正相关关系(r分别为0.9 304, 0.8 680, P<0.001).
核因子-κB作为调节细胞基因转录的关键因子, 具有广泛的生物学活性, 在机体免疫应答、炎症反应及细胞的生长调控等方面发挥重要的作用[17-22], 已成为众多领域研究的热点. 但其与溃疡性结肠炎关系的研究国内尚未见报道. 我们采用三硝基苯磺酸(TNBS)诱导形成大鼠溃疡性结肠炎, 并以不同剂量TNBS制备病情轻重不-的疾病模型, 通过免疫组化染色检测NF-κB蛋白的表达, 结果发现, 在大鼠溃疡性结肠炎模型组中, NF-κB的表达水平明显增强, 且在大剂量TNBS的模型Ⅱ组尤为明显. 提示NF-κB可能在溃疡性结肠炎的发病机制中起重要作用.
溃疡性结肠炎是肠道炎性疾病, 肠道局部炎性损伤是其重要病理改变, 以大量炎性细胞浸润肠壁和持续活化为特征. 现已证实[9-15], 多种炎性因子表达异常参与了溃疡性结肠炎的病理损伤过程, 而TNF-α和ICAM-1则是较受关注的因子之一, 且在TNF-α和ICAM-1基因序列上均含有NF-κB的结合位点. TNF-α是来源于单核巨噬细胞的细胞因子, 具有广泛的前炎性活性, 能诱导黏附分子在上皮细胞及淋巴细胞的表达, 诱导趋化因子产生, 介导炎性白细胞向炎症局部聚集[26,27]; ICAM-1可介导炎性白细胞与血管内皮细胞、上皮细胞稳定黏附, 使炎性细胞定位于炎症部位[28-30]. 二者在炎性细胞浸润及肠道炎症形成过程中起重要作用. 我们的研究表明, 与正常组和生理盐水组大鼠相比, 模型组大鼠肠组织中TNF-α和ICAM-1阳性表达率和阳性细胞密度显著增高(P<0.01), 且模型Ⅱ组高于模型Ⅰ组, 说明TNF-α和ICAM-1的异常与溃疡性结肠炎的发病有关, 且其表达与疾病病情严重程度也有关系, 这与过去的结论-致[8,31-34].
NF-κB最常见的形式是由P50与P65亚基组成的异二聚体, 在静息状态下, NF-κB与抑制因子I κB单体耦联, 以无活性的形式存在于细胞质中, 在外界多种因素刺激下, I κB磷酸化后降解, NF-κB得以释放并移入核内与靶基因序列上特定的结合位点结合, 启动或调节基因转录[22,35]; 其参与多种与机体免疫炎症反应密切相关的基因转录, 如细胞因子(IL-2、IL-6、IL-8及TNF-α等), 黏附分子(ICAM-1、VCAM、ELAM、E-selection等)以及T细胞受体和INF-γ等[17-23]. 这些因子在炎性细胞的活化、趋化、浸润等过程中有重要作用. Jobin et al[36]在大鼠肠上皮细胞系CEI-6细胞中发现TNF-α可以激活NF-kB而促使ICAM-1 mRNA和蛋白表达上升, 而采用蛋白酶抑制剂MG-132或ALLN阻断 IκB降解过程, 可明显降低NF-κB活性和ICAM-1基因表达. 有研究证实, 脂多糖刺激NF-κB活化后可促进单核细胞分泌IL-1、TNF-α增多, 后者通过正反馈作用又激活单核细胞的NF-κB使自身分泌量进一步增加, 引起级联反应[37]. 我们的结果发现, 在大鼠溃疡性结肠炎肠组织中NF-κB表达水平与TNF-α、ICAM-1阳性表达呈正相关关系, 因此我们推测, NF-κB可能通过调控炎性因子TNF-α、ICAM-1基因转录, 参与了溃疡性结肠炎的炎性损害过程.
本实验结果还显示, 溃疡性结肠炎大鼠肠组织NF-κB表达水平与肠道病理损害的大体形态和组织学评分指数及髓过氧化物酶(MPO)含量呈显著性正相关关系, 而MPO是中性粒细胞中含量较高的一种酶, 在单核细胞和巨噬细胞中也少量存在, 其含量可间接反映组织炎性细胞的浸润程度[38,39], 提示溃疡性结肠炎肠组织NF-κB表达量可反映肠组织的病理损害程度, 对于溃疡性结肠炎病情的评估及治疗效果的判断, 可能具有潜在的指导意义.
总之, 我们的研究提示, NF-kB与溃疡性结肠炎的发病关系密切, 对肠组织中炎性因子TNF-α和ICAM-1等基因转录的调控可能是其作用的机制之一, 其表达量也可作为评价肠道病理损害的指标之一. 但NF-κB在溃疡性结肠炎中活化机制以及对炎性因子表达调控的具体过程等均有待于进一步的研究.
编辑: N/A
| 1. | Jiang XL, Cui HF. An analysis of 10218 ulcerative colitis cases in China. World J Gastroenterol. 2002;8:158-161. [PubMed] [DOI] |
| 2. | Xu G, Tian KL, Liu GP, Zhong XJ, Tang SL, Sun YP. Clinical significance of plasma D-dimer and von Willebrand factor levels in patients with ulcer colitis. World J Gastroenterol. 2002;8:575-576. [PubMed] [DOI] |
| 3. | Cui HF, Jiang XL. Treatment of corticosteroid-resistant ulcerative colitis with oral low molecular weight heparin. World J Gastroenterol. 1999;5:448-450. [PubMed] [DOI] |
| 4. | Salupere R. Inflammatory bowel disease in Estonia: a prospective epidemiologic study 1993-1998. World J Gastroenterol. 2001;7:387-388. [DOI] |
| 5. | Kirsner JB. Historical origins of current IBD concepts. World J Gastroenterol. 2001;7:175-184. [PubMed] [DOI] |
| 6. | Wu HG, Zhou LB, Shi DR, Liu SM, Liu HR, Zhang BM, Chen HP, Zhang LS. Morphological study on colonic pathology in ulcerative colitis treated by moxibustion. World J Gastroenterol. 2000;6:861-865. [PubMed] [DOI] |
| 7. | MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2-9. [PubMed] [DOI] |
| 8. | Xia B, Guo HJ, Crusius J, Deng CS, Meuwissen S, Pena A. In vitro production of TNF-alpha,IL-6 and sIL-2R in Chinese patients with ulcerative colitis. World J Gastroenterol. 1998;4:252-255. [PubMed] [DOI] |
| 9. | Monteleone G, MacDonald TT. Manipulation of cytokines in the management of patients with inflammatory bowel disease. Ann Med. 2000;32:552-560. [PubMed] [DOI] |
| 10. | Funakoshi K, Sugimura K, Anezaki K, Bannai H, Ishizuka K, Asakura H. Spectrum of cytokine gene expression in intestinal mucosal lesions of Crohn's disease and ulcerative colitis. Digestion. 1998;59:73-78. [PubMed] [DOI] |
| 11. | Wu HG, Zhou LB, Pan YY, Huang C, Chen HP, Shi Z, Hua XG. Study of the mechanisms of acupuncture and moxibustion treatment for ulcerative colitis rats in view of the gene expression of cytokines. World J Gastroenterol. 1999;5:515-517. [PubMed] [DOI] |
| 12. | Inoue S, Matsumoto T, Iida M, Mizuno M, Kuroki F, Hoshika K, Shimizu M. Characterization of cytokine expression in the rectal mucosa of ulcerative colitis: correlation with disease activity. Am J Gastroenterol. 1999;94:2441-2446. [PubMed] [DOI] |
| 13. | Brown KA, Back SJ, Ruchelli ED, Markowitz J, Mascarenhas M, Verma R, Piccoli DA, Baldassano RN. Lamina propria and circulating interleukin-6 in newly diagnosed pediatric inflammatory bowel disease patients. Am J Gastroenterol. 2002;97:2603-2608. [PubMed] [DOI] |
| 14. | Vainer B, Nielsen OH. Changed colonic profile of P-selectin, platelet-endothelial cell adhesion molecule-1 (PECAM-1), intercellular adhesion molecule-1 (ICAM-1), ICAM-2, and ICAM-3 in inflammatory bowel disease. Clin Exp Immunol. 2000;121:242-247. [PubMed] [DOI] |
| 15. | Kolios G, Petoumenos C, Nakos A. Mediators of inflammation: production and implication in inflammatory bowel disease. Hepatogastroenterology. 1998;45:1601-1609. [PubMed] |
| 16. | Stoyanova II, Gulubova MV. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002;104:185-192. [PubMed] [DOI] |
| 17. | May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80-88. [PubMed] [DOI] |
| 18. | Chen FE, Huang DB, Chen YQ, Ghosh G. Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature. 1998;391:410-413. [PubMed] [DOI] |
| 19. | Lee JI, Burckart GJ. Nuclear factor kappa B: important transcription factor and therapeutic target. J Clin Pharmacol. 1998;38:981-993. [PubMed] [DOI] |
| 20. | Baldwin AS Jr. Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3-6. [PubMed] [DOI] |
| 21. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. [PubMed] [DOI] |
| 22. | Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225-260. [PubMed] [DOI] |
| 23. | Neurath MF, Becker C, Barbulescu K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut. 1998;43:856-860. [PubMed] [DOI] |
| 24. | Butzner JD, Parmar R, Bell CJ, Dalal V. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat. Gut. 1996;38:568-573. [PubMed] [DOI] |
| 25. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [PubMed] [DOI] |
| 26. | Obermeier F, Kojouharoff G, Hans W, Schölmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238-245. [PubMed] [DOI] |
| 27. | Lampinen M, Carlson M, Sangfelt P, Taha Y, Thörn M, Lööf L, Raab Y, Venge P. IL-5 and TNF-alpha participate in recruitment of eosinophils to intestinal mucosa in ulcerative colitis. Dig Dis Sci. 2001;46:2004-2009. [PubMed] [DOI] |
| 28. | Woywodt A, Ludwig D, Neustock P, Kruse A, Schwarting K, Jantschek G, Kirchner H, Stange EF. Mucosal cytokine expression, cellular markers and adhesion molecules in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1999;11:267-276. [PubMed] [DOI] |
| 29. | Sans M, Panés J, Ardite E, Elizalde JI, Arce Y, Elena M, Palacín A, Fernández-Checa JC, Anderson DC, Lobb R. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterology. 1999;116:874-883. [PubMed] [DOI] |
| 30. | Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Adhesion molecule and proinflammatory cytokine gene expression in hepatic sinusoidal endothelial cells following cecal ligation and puncture. World J Gastroenterol. 2001;7:128-130. [PubMed] [DOI] |
| 31. | Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999;34:66-74. [PubMed] [DOI] |
| 32. | Louis E. The immuno-inflammatory reaction in Crohn's disease and ulcerative colitis: characterisation, genetics and clinical application. Focus on TNF alpha. Acta Gastroenterol Belg. 2001;64:1-5. [PubMed] |
| 33. | Brandtzaeg P. Inflammatory bowel disease: clinics and pathology. Do inflammatory bowel disease and periodontal disease have similar immunopathogeneses? Acta Odontol Scand. 2001;59:235-243. [PubMed] [DOI] |
| 34. | Vainer B, Nielsen OH, Horn T. Comparative studies of the colonic in situ expression of intercellular adhesion molecules (ICAM-1, -2, and -3), beta2 integrins (LFA-1, Mac-1, and p150,95), and PECAM-1 in ulcerative colitis and Crohn's disease. Am J Surg Pathol. 2000;24:1115-1124. [PubMed] [DOI] |
| 35. | Cong B, Li SJ, Yao YX, Zhu GJ, Ling YL. Effect of cholecystokinin octapeptide on tumor necrosis factor alpha transcription and nuclear factor-kappaB activity induced by lipopolysaccharide in rat pulmonary interstitial macrophages. World J Gastroenterol. 2002;8:718-723. [PubMed] [DOI] |
| 36. | Jobin C, Hellerbrand C, Licato LL, Brenner DA, Sartor RB. Mediation by NF-kappa B of cytokine induced expression of intercellular adhesion molecule 1 (ICAM-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut. 1998;42:779-787. [PubMed] [DOI] |
| 37. | Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3-9. [PubMed] [DOI] |
| 38. | Zhu F, Qian J, Pan G, Quan S. [The effect of interleukin-1 beta interleukin-8 in the pathogenesis of experimental colitis and evaluation of interleukin-1 receptor antagonist therapy]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1998;20:388-394. [PubMed] |
| 39. | Zheng L, Gao ZQ, Wang SX. A chronic ulcerative colitis model in rats. World J Gastroenterol. 2000;6:150-152. [PubMed] [DOI] |