修回日期: 2002-11-01
接受日期: 2002-11-20
在线出版日期: 2003-02-15
目的: 探讨多位点自剪切核酶及突变核酶对细胞内HBV mRNA的切割作用.
方法: 构建5个不同的多位点核酶及突变核酶的真核表达载体, 将他们分别与乙型肝炎病毒全基因序列共转染HepG2细胞, 用ELISA, 共聚焦定量及图像分析的方法观察多位点核酶在细胞内对HBV mRNA切割作用.
结果: 构建的真核表达载体在细胞内确可表达出多位点核酶, 核酶及突变核酶在细胞内对HBV基因的表达均有抑制作用, 不同表达载体的抑制率不同, 以tRNA启动子的表达载体抑制效率最高, 达81%, 突变核酶亦有部分反义RNA的抑制效果.
结论: 抗乙型肝炎病毒核酶在细胞内可抑制HBV基因的表达, 不同表达载体其核酶的表达效率不同.
引文著录: 李谨革, 连建奇, 贾战生, 冯志华, 聂青和, 王九平, 黄长形, 白雪帆. 不同载体表达核酶对HBV mRNA细胞内表达的阻断作用. 世界华人消化杂志 2003; 11(2): 161-164
Revised: November 1, 2002
Accepted: November 20, 2002
Published online: February 15, 2003
AIM: To study the activity of ribozymes with multiple cleavage sites and mutated ribozymes on expression of HBV mRNA in HepG2 cells.
METHODS: The triple ribozymes and two cis-ribozymes or two mutated ribozymes were inserted, respectively, into five kinds of eukaryotic plasmids, which were cotransfected into the HepG2 cells with p1.2Ⅱplasmid carring genome of adv-subtype HBV. Cleavage effect of ribozymes on HBeAg and HBcAg were detected by ELISA and laser confocal imaging technique.
RESULTS: The transfected HepG2 cells expressed the expected ribozyme and muta-ribozyme. Intracellular level of HBeAg was surpressed variably with variety of ribozymes. The ribozyme plasmid with tRNA promoter demonstrated the highest inhibitory rate at 81% for suppression HBeAg expression.
CONCLUSION: The ribozymes exert varied inhibitory effect on the expression of HBV in HepG2 cells depending on kinds of eukaryotic expressing plasmids.
- Citation: Li JG, Lian JQ, Jia ZS, Feng ZH, Nie QH, Wang JP, Huang CX, Bai XF. Effect of ribozymes on inhibiting expression of HBV mRNA in HepG2 cells. Shijie Huaren Xiaohua Zazhi 2003; 11(2): 161-164
- URL: https://www.wjgnet.com/1009-3079/full/v11/i2/161.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i2.161
HBV (hepatitis B virus), 是一种严重危害人类健康的病原体, 能够引起急、慢性乙型肝炎[1-7]. 而且慢性HBV携带者发生肝癌的可能性是正常人的100倍. 目前的研究确信HBV是少数几种致癌病毒之一, HBV的感染、复制等生命活的依赖基因组的转录和表达. 同时HBcAg在细胞内达到一定量后, 可取代细胞染色体中组蛋白, 而使宿主细胞的正常基因表达发生紊乱. 而且HBcAg也免疫因子作用的靶位点[8].
目前常用的药物治疗方法无法阻断细胞中HBV的表达, 起到细胞内的保护作用, 核酶做为一种新效法已显出明显优势[9-32]. 前期的研究中, 我们采用核酶对HBV表达进行阻断, 已取得一定效果, 其抑制率低于66%. 为提高核酶的剪切活性, 我们采用了自剪切载体表达3个位点核酶, 且对照组使用突变核酶, 用不同的载体及启动子表达核酶, 观察核酶对相应蛋白的抑制作用.
p1.2Ⅱ(含乙型肝炎病毒基因全序列), 由连建奇博士惠赠, pcDNA3由冯志华博士惠赠. pCI由军事医学科学院王海涛教授惠赠. pBBS212及pCEp由本室保存. HepG2细胞由本校病理教研室惠赠. DMEM, Lipofectamine 2 000购自Gibco公司. ELISA检测试剂盒购自科华公司. 各种抗体购自DAKO公司.
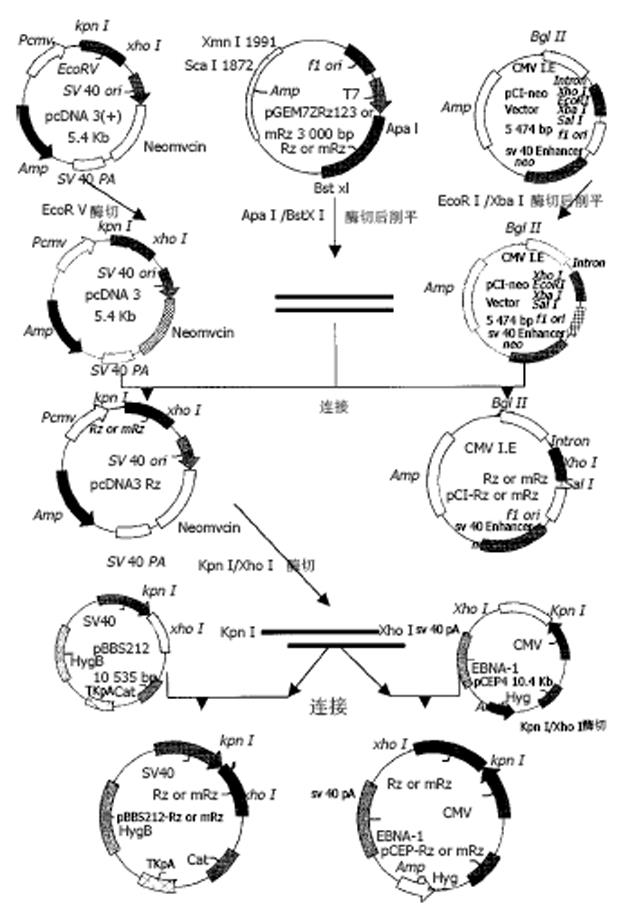
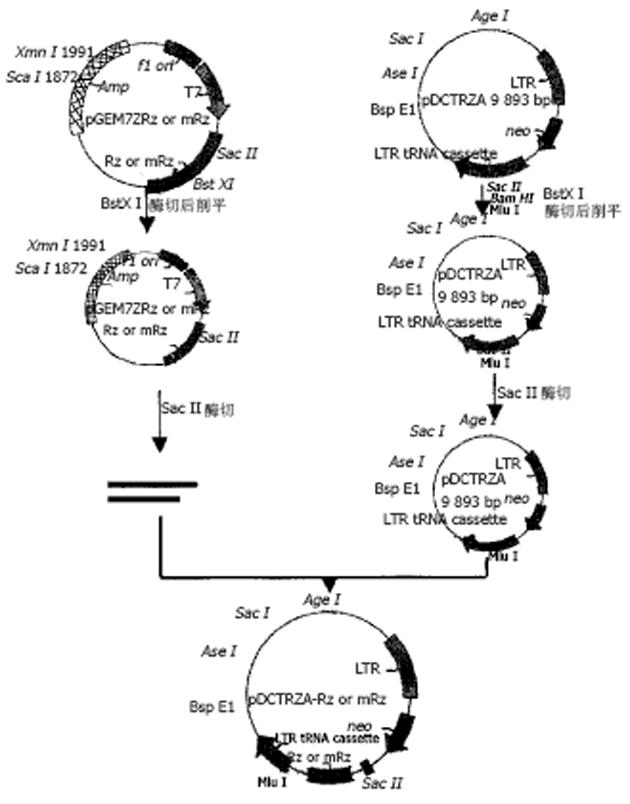
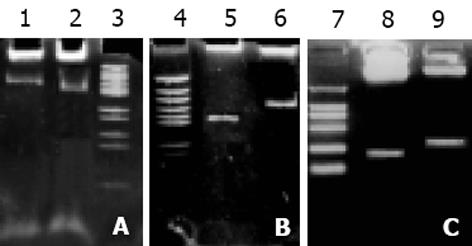
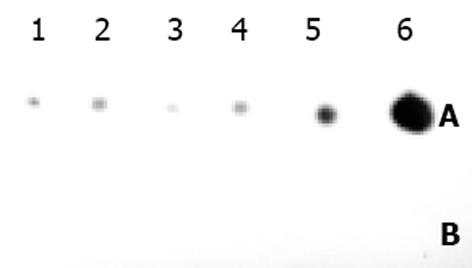
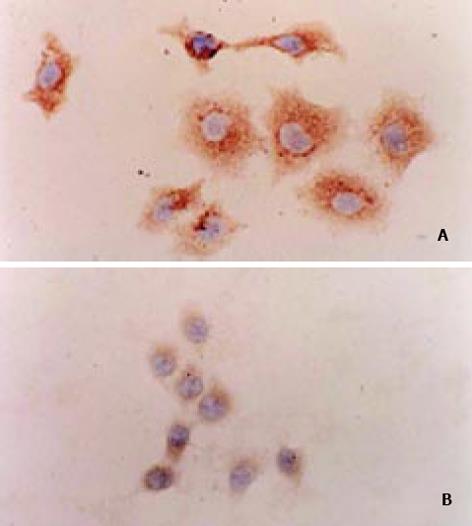
表达自载切核酶的不同真核表达载体的构建 pcDNA3-Rz123 pcDNA3-mRz12的连接, 将核酶及突变核酶, 用ApaI/Bst XI双酶切, 回收270 bp左右的DNA片段, pcDNA用EcoRⅤ酶切, 回收做为载体. 回收的片段经T4DNA聚合酶补平后, 将二者进行连接, 转化后挑克隆进行鉴定, 鉴定采用XbaI/HindⅢ双酶切或XbaI单酶切(图1). pCI-Rz123 及pCI-mRz123的连接, 将pGEMRz123及pGEM-mRz12用ApaI/BstXI双酶切. 回收补平, pCI用EcoRI及XbaI双酶切后回收补平、连接、转化, 挑取克隆, 用XhoI+SalI双酶切鉴定大小及用XbaI+SalI鉴定方向. pBBS212-Rz123及pBBS212-mRz12的连接, 将pBBS212及pcDNA3-Rz123及pcDNA3-mRz12均用Kpn I及Xho I进行双酶切, 回收片段及载体, 双粘端连接, 转化. 挑取克隆, 鉴定用Kpn I及Xho I进行双酶切. pCEp-Rz123 pCEp-mRz12的连接, 将pCEp及pcDNA3-Rz123及pcDNA3-mRz12均用Kpn I及Xho I进行双酶切, 回收片段及载体, 进行双粘端连接、转化, 挑取克隆, 鉴定用Kpn I及Xho I进行双酶切. pDCTRZA-Rz123 与pDCTRZA-mRz12的连接, 将pGEM-Rz123 及pGEMRz-mRz12, 用BstX I单切后切胶回收、补平, 用SacⅠ酶切后回收270 bp及200 bp左右的DNA片段. 将pDCTRZA质粒用BamH I单切后切胶回收、补平, 用Sac Ⅱ酶切酶回收载体DNA, 将载体与片段进行连接、转化. 挑取克隆, 鉴定用Sac Ⅱ、Mlu I进行双酶切(图2). 质粒DNA转染细胞前16-20 h, 用胰酶消化HepG2细胞, 接种于6孔培养板(1×105/孔)37℃, 待细胞生长至50%-70%融合时, 取7.5 mg核酶及突变核酶质粒DNA及7.5 mg p1.2Ⅱ质粒DNA溶于100 mL无血清DMEM中, 混匀; 取Lipofectamine 20 008 mL加无血清DMEM中, 混匀. 缓慢混合, 室温25 min, 加无血清DMEM800 mL至总体积1 mL, 混匀. 用无血清DMEM洗细胞1次, 加入上述转染液于培养板(1 mL/well), 37℃培养16 h, 换完全培养液, 传代, 48 h后更换选择性培养液, 筛选出阳性克隆后, 混合克隆, 次日收集细胞. 细胞用冰浴的PBS缓冲液冲洗3次, 加1 mL含10 mmol/LEDTA的PBS缓冲液消化细胞, 加入1-10 mL PBS悬浮细胞, 计数离心, 去除上清, 悬浮细胞于0.25 mmol/L Tris-HCl (pH7.5) (100 mL/106细胞), 上清即为胞质裂解液, 用于测定HBeAg、ELISA检测按说明书进行. A值在450 nm下读取. 结果均以阳性孔A值/阴性孔A值(P/N)表示, 抑制率按下式计算: 抑制率 = (实验孔P/N值-对照孔P/N值)÷(对照孔P/N值-2.1)×100%免疫荧光及激光共聚焦的检测转染的细胞爬片后固定, 滴加HBcAg抗体IgG, 37℃, 30min, 洗涤5次, 滴加荧光抗体37℃, 30 min, 吹干, 封片, 镜检, 用激光共聚焦显微镜观测, 定量. 免疫细胞化学用SABC法检测(参见说明书), 共转染HepG2细胞染色结果判定, 呈棕色者为阳性, 不着色者为阴性, 依据其着色的深浅, 代表HBcAg表达的量的多少, 不同载体转染各选择5份标本, 40倍镜下随机各选择50个细胞, 测定其胞度的灰度, 计算其均数及标准差, 所有结果均以SPLM软件进行t检测. 细胞的总RNA用异硫氰酸胍法提取, 用32P标记单链DNA的5′末端, 打点杂交方法参见说明书.
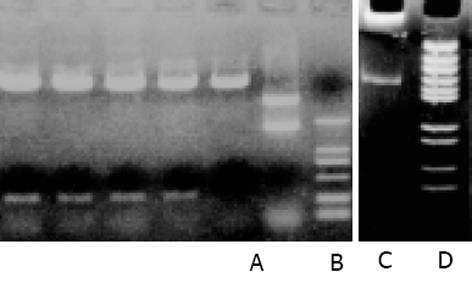
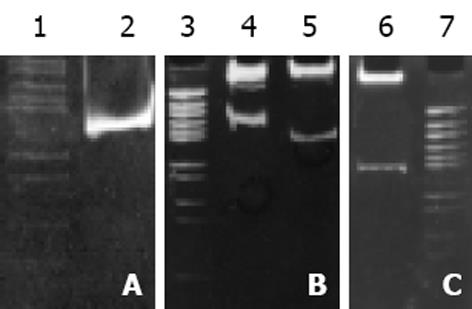
pcDNA3-Rz123与pcDNA3-mRz12质粒, 用不同的单酶或双酶酶切鉴定结果与预想结果相同, 说明质粒构建成功(图3). pCI-Rz123 及pCI-mRz12质粒, pBBS212-Rz123及pBBS212-mRz12, pCEp-Rz123及pCEp-mRz12, pDCTRZA-Rz123 及pDCTRZA-mRz12, 用不同的单酶或双酶切鉴定结果与预计结果相吻合, 证明构建质粒成功(图4, 5).
在将p1.2Ⅱ与多个核酶的不同载体以及突变核酶, 空载体分别转染HepG2细胞, 转染1 wk后胞质中可见到HBeAg的表达. 待筛选出阳性克隆后, 取胞质裂解液进行ELISA测定. 由表中可见, 不同的载体表达核酶, 对HBeAg的表达有不同的抑制率, 以pDCTRZA为最高, 为81%, 其次为pBBS212 76%, 最低为pCEp, 且突变核酶亦有一定的抑制率, 说明其具有部分反义RNA的功能(表1, 图6).
| 分组 | pCDNA3 | pCI | pBBS212 | pCEP | pDCTRZA |
| 核酶组 | 2.6±1.3(0.73) | 2.9±0.7(0.61) | 2.5±0.4(0.76) | 3.1±0.4(0.55) | 2.6±1.1(0.81) |
| 突变核酶组 | 3.6±1.0(0.18) | 3.7±0.7(0.21) | 3.4±0.1(0.26) | 3.9±1.0(0.21) | 3.8±1.0(0.39) |
| 空载体组 | 3.9±0.1 | 4.2±0.1 | 3.9±1.10 | 4.4±0.1 | 4.9±0.3 |
取突变组及核酶组细胞分别进行免疫荧光测定, 荧光强度差异明显. 将其进行共聚焦定量, 以下列公式计算: 抑制率 = 1-核酶组像素密度/突变核酶组像素密度, 经计算, 其最高抑制率可达73.2%.
取突变组、空载体组及核酶组两种细胞抗原表达有明显差异(图7). 将此细胞进行图像分析, 亦表明二者有差异.
近年来, 在细胞内应用核酶抑制基因表达已取得了令人瞩目的成果, 尤其抗HIV核酶已在CD4+淋巴细胞中表达, 并将进行回输人体进行研究. 因此尽管核酶的酶效率较低, 比蛋白质低几个数量级, 但在细胞和活体研究中, 却发现他可以降低靶RNA 90%以上. 可见核酶用于抑制基因表达和基因治疗有着广阔的前景. 乙型肝炎病毒(HBV)的复制必须经过由细胞的RNA多聚酶转录成多拷贝的3.5 kb RNA, 以这一RNA前基因组为模板, 通过逆转录, 自DR1区开始合成负链DNA, 因此前基因组mRNA是关键的一步, 所设计的核酶如能正确切割mRNA, 则可抑制乙型肝炎病毒的复制和表达, 从而起到胞内免疫的作用. 到目前为止, 关于细胞内核酶抗HBV的研究尚不多[32], Beck所用核酶没有在细胞内做到有活性的表达, 而Welch则用串联核酶表达, 做到最高抑制率83%, 南非学者也用荧光蛋白来检测细胞内核酶对HBV的抑制作用, 证实确可抑制HBV的表达.
本室前期的工作亦证实对HBV C区基因的表达有明显的抑制作用. 同时在前期工作的基础上, 我们增加了自剪切序列及核酶的数量, 用自剪切来包装核酶, 避免其两侧的附加序列影响到核酶的切割活性, 使切割核酶与靶RNA作用后更易脱落下来, 进行新一轮的切割. 增加核酶数量则使其能在不同的位点破坏靶RNA, 避免病毒变异造成的单一核酶切割作用的丧失, 也可避免结合蛋白掩盖切割位点. 细胞内核酶的表达, 关键在于启动子的选择, Cotton将核酶基因克隆在蛙蟾tRNA met及密码环中, 提高了核酶的转录水平. Baier利用tRNA启动子可使核酶基因高效表达, 同时tRNA的结构稳定, 可抵抗核酸酶的降解, 因此被认为是细胞内表达核酶的理想启动子. 本研究即将核酶克隆于含有tRNA启动子的真核载体中. 本研究的结果也印证了上述理论, 杂交证实转染细胞中有核酶的mRNA存在, ELISA等方法检测发现核酶及突变核酶对HBV的表达均有抑制作用. pcDNA3, pCI, pBBS212, pCEp, pDCTRZA所达核酶对HBV C基因抑制率为73%, 61%, 76%, 55%, 81%. 最高者为pDCTRZA即tRNA启动子所启动表达的核酶. 这也说明核酶作为一种抗HBV的手段是可行的.
编辑: N/A
| 1. | Rabe C, Pilz T, Klostermann C, Berna M, Schild HH, Sauerbruch T, Caselmann WH. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:208-215. [PubMed] [DOI] |
| 2. | Zhuang L, You J, Tang BZ, Ding SY, Yan KH, Peng D, Zhang YM, Zhang L. Preliminary results of Thymosin-a1 versus interferon-alpha-treatment in patients with HBeAg negative and serum HBV DNA positive chronic hepatitis B. World J Gastroenterol. 2001;7:407-410. [PubMed] [DOI] |
| 3. | Li JG, Zhou YX, Lian JQ. Intracellular applicaton of two-unit ribozyme gene against hepatitis B virus. Zhonghua Neike Zazhi. 2000;39:27-30. |
| 4. | Shan Y, Xiong SS, Liu X, Zhao M, Ba QJ, Zhou LJ. Comparison of human leukocyte interferon and recombinant interferon alpha 1 in the treatment of chronic hepatitis B. Xin Xiaohuabingxue Zazhi. 1997;5:40-41. [DOI] |
| 5. | Liu WE, Tan DM, Fan XG, Ouyang K, Zhang Z. Role of autoimmune reaction in pathogenesis of patients with chronic HCV infection. Shijie Huaren Xiaohua Zazhi. 1999;7:120-121. [DOI] |
| 6. | Xie Q, Guo Q, Zhou XQ, Gu RY. Effect of adenine arabinoside monophosphate coupled to lactosaminated human serum albumin on duck hepatitis B virus. Shijie Huaren Xiaohua Zazhi. 1999;7:125-126. [DOI] |
| 7. | Xu KC, Wei BH, Yao XX, Zhang WD. Recent therapy for chronic hepatitis B by combined traditional Chinese and Western medicine. Shijie Huaren Xiaohua Zazhi. 1999;7:970-974. [DOI] |
| 8. | Pan X, Ke CW, Pan W, Wu WB, Zhang B, He X, Cao GW, Qi ZT. Construction of eukaryotic expression vector carrying IFN- gene under control of human HBV promoter. Shijie Huaren Xiaohua Zazhi. 2000;8:520-523. [DOI] |
| 9. | Worman HJ, Feng L, Mamiya N. Molecular biology and the diagnosis and treatment of liver diseases. World J Gastroenterol. 1998;4:185-191. [PubMed] [DOI] |
| 10. | Zhoug S, Wen SM, Zhang DF, Wang QL, Wang SQ, Ren H. Sequencing of PCR amplified HBV DNA pre-c and c regions in the 2.2.15 cells and antiviral action by targeted antisense oligonucleotide directed against sequence. World J Gastroenterol. 1998;4:434-436. [PubMed] [DOI] |
| 11. | Yang SM, Zhou H, Chen RC, Wang YF, Chen F, Zhang CG, Zhen Y, Yan JH, Su JH. Sequencing of p53 mutation in established human hepatocellular carcinoma cell line of HHC4 and HHC15 in nude mice. World J Gastroenterol. 1998;4:506-510. [PubMed] [DOI] |
| 12. | Shi JJ, Miao F, Liu FL. Therapeutic effect of medicinal herbs and western drugs on hepatitis B virus. World J Gastroenterol. 1998;4:61-62. [DOI] |
| 13. | Qiu AG, Qiu RB, Miao Y, Fu ZL, Zhang YR, Zheng YQ, Hong YS, Wu BS, Jiang YP, Qian CF. Clinical study on therapeutic effect of three cycle natural therapy on chronic hepatitis B and C. World J Gastroenterol. 1998;4:82. [DOI] |
| 14. | Zhu Y, Wang YL, Shi L. Clinical analysis of the efficacy of interferon alpha treatment of hepatitis. World J Gastroenterol. 1998;4:85-86. [DOI] |
| 15. | Wang Y, Liu H, Zhou Q, Li X. Analysis of point mutation in site 1896 of HBV precore and its detection in the tissues and serum of HCC patients. World J Gastroenterol. 2000;6:395-397. [PubMed] [DOI] |
| 16. | Ma CH, Sun WS, Zhang LN, Ding PF. Inhibitory effect of antisense oligodeoxynucleotides complementary to HBV on HepG2.2.15 cell line. World J Gastroenterol. 2000;6:72. |
| 17. | Gao XW, Jia SY, Liu XM. BCG vaccine combined with dipyridamole in the treatment of HBV infection. World J Gastroenterol. 2000;6:76. |
| 18. | Bo AH, Tian CS, Xue GP, Du JH, Xu YL. Morphology of immune and alcoholic liver diseases in rats. Shijie Huaren Xiaohua Zazhi. 2001;9:157-160. [DOI] |
| 19. | Hao CQ, Zhou YX, Feng ZH, Li JG, Jia ZS, Wang PZ. Construction, identification and expression of framework plasmid pAd. HCV- C of adenovirus expression vector of HCV C. Shijie Huaren Xiaohua Zazhi. 2001;9:635-639. [DOI] |
| 20. | Tu SP, Wu YL, Sun J, He Q, Ke YM, Fu H, Yuan YZ, Jiang SH. Disinfecting effect of electrolyzed acid water on gastroendoscope. Shijie Huaren Xiaohua Zazhi. 2001;9:874-876. [DOI] |
| 21. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] [DOI] |
| 22. | Ghaneh P, Slavin J, Sutton R, Hartley M, Neoptolemos JP. Adjuvant therapy in pancreatic cancer. World J Gastroenterol. 2001;7:482-489. [PubMed] [DOI] |
| 23. | Spangenberg HC, Wands JR. Ribozymes and hepatitis B virus. J Gastroenterol Hepatol. 2001;16:1084-1085. [PubMed] [DOI] |
| 24. | Li JG, Zhou YX, Li JQ, Feng ZH, Li GY. Preliminary study on the inhibition of HBeAg expression in cells by ribozyme. Zhonghua Chuanranbing Zazhi. 1999;17:152-154. |
| 25. | Li JG, Zhou YX, Li JQ, Jia ZS, Feng ZH. Preliminary study on ribozyme inhibit gene expression. Zhonghua Ganzangbing Zazhi. 1999;7:181. |
| 26. | Li JG, Zhou YX, Li JQ, Feng ZH. Intercellular application two-unit ribozyme gene against hepatitis B virus. Jiefangjun Yixue Zazhi. 1999;24:99-101. |
| 27. | Feng Y, Kong Y, Wang Y, Qi G. Antiviral Activity of a Hammerhead Ribozyme against HBV in HepG2.2.15 Cells. Shengwuhuaxue Yu Shengwuwuli Xuebao (Shanghai). 2002;34:204-20828 Weinberg M, Passman M, Kew M, Arbuthnot P. Hammerhead ribozyme-mediated inhibition of hepatitis B virus X gene expression in cultured cells. J Hepatol 2000; 33: 142-151. [PubMed] [DOI] |
| 28. | Li JG, Zhou YX, Lian JQ, Jia ZS, Feng ZH. Inhibitory effect of ribozyme on HBeAg in human HCC cells. Shijie Huaren Xiaohua Zazhi. 1999;7:28-30. [DOI] |
| 29. | Zheng WC, Lu CD, Kong YY, Wang Y, Qi GR. Hammerhead Ribozymes Suppress HBV(adr) in HepG2 Cells. Shengwuhuaxue Yu Shengwuwuli Xuebao (Shanghai). 2001;33:25-29. |
| 30. | Song YH, Lin JS, Liu NZ, Kong XJ, Xie N, Wang NX, Jin YX, Liang KH. Anti-HBV hairpin ribozyme-mediated cleavage of target RNA in vitro. World J Gastroenterol. 2002;8:91-94. [PubMed] [DOI] |
| 31. | Wen SJ, Xiang KJ, Huang ZH, Zhou R, Qi XZ. Construction of HBV-specific ribozyme and its recombinant with HDV and their cleavage activity in vitro. World J Gastroenterol. 2000;6:377-380. [PubMed] [DOI] |