修回日期: 2002-11-01
接受日期: 2002-11-20
在线出版日期: 2003-02-15
目的: 探讨体外分离培养的人体胎盘滋养层细胞感染丙型肝炎病毒(HCV)的可能性, 以及滋养层细胞感染HCV后的超微结构改变.
方法: 采用胰蛋白酶消化法及Percoll密度梯度分离法分离培养人胎盘组织中滋养层细胞后, 以HCV RNA阳性血清对滋养层细胞进行体外感染试验, 应用RT-PCR法定性及定量检测感染后细胞培养上清中HCV RNA, 透射电镜观察HCV感染后滋养层细胞的超微结构改变, 并用激光共聚焦技术观察免疫荧光染色后HCV NS5在滋养层细胞中的定位.
结果: HCV感染后的细胞培养上清中可间断检测到HCV RNA, 而对照组始终未检出; 激光共聚焦观察显示HCV NS5主要定位于细胞核周; HCV感染后滋养层细胞超微结构发生了明显改变, 主要表现为溶酶体大量增生, 粗面内质网增生, 脂滴减少, 出现空泡状结构, 并可观察到病毒样颗粒.
结论: HCV可以感染滋养层细胞, 并导致滋养层细胞的超微结构发生类似黄病毒科病毒感染后的改变.
引文著录: 程勇前, 聂青和, 周永兴, 黄晓峰, 罗红, 杨华光. 透射电镜及激光共聚焦技术观察体外丙型肝炎病毒感染的人胎盘滋养层细胞. 世界华人消化杂志 2003; 11(2): 151-156
Revised: November 1, 2002
Accepted: November 20, 2002
Published online: February 15, 2003
AIM: To investigate whether the cultured trophoblastic cells can be infected with hepatitis C virus (HCV) and observe the ultrastructural features of infected cells.
METHODS: Human placentae were digested with trypsin and then centrifuged with percoll density gradient to obtain trophoblastic cells, and then incubated in HCV positive serum. The HCV RNA in HCV infected syncytiotroblasts was quantitated with RT-PCR. Ultrastructural characteristics of infected syncytiotroblasts were observed with transmission electron microscope.
RESULTS: HCV RNA was detected in supernatant of the cultured medium during 40 day periods of incubation. The antibody of HCV NS5 was observed around the nucleus with confocal microscope. The Ultrastructure of infected throphotoblast cells differed obviously from that of normal cells, and manifested with hyperplasia of lysosomes and rough endoplasmic, appearance of vacuoles and virus-like particles, and decreased lipid droplets.
CONCLUSION: Trophoblastic cells could be infected by HCV, and the cellular ultrastructure changed dramatically following infection of HCV.
- Citation: Cheng YQ, Nie QH, Zhou YX, Huang XF, Luo H, Yang HG. Ultrastructure characteristics of HCV infected human trophoblast cells in culture. Shijie Huaren Xiaohua Zazhi 2003; 11(2): 151-156
- URL: https://www.wjgnet.com/1009-3079/full/v11/i2/151.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i2.151
HCV主要经血液或血制品传播, 随着对血制品检测的加强及检测技术的提高, HCV经输血传播的发生率已大大降低[1]. 现研究认为HCV存在母婴传播[2-8], HCV感染的母亲中, HCV母婴传播发生率大约为5%左右[9-13], 但目前尚无有效的方法阻断HCV的母婴传播, 因此HCV的母婴传播将可能成为未来HCV感染的重要途径[14]. 我国丙型肝炎较常见, 对人类健康危害很大, 在目前尚无特效药物治疗HCV感染, 以及丙型肝炎疫苗尚难以用于人体预防HCV感染的情况下[15-33], 阻断HCV母婴传播的研究工作显得十分重要.
目前有关HCV母婴传播的研究多数为流行病学调查, 关于HCV如何在母婴间进行传播的具体机制尚不清楚, 可能为宫内传播、出生时传播和出生后通过母乳、唾液等, 而宫内感染可能是HCV母婴传播的主要机制之一. 胎盘是胎儿与母体之间进行物质交换的重要器官, 由滋养层细胞、毛细血管内皮细胞以及二者之间基膜所构成的胎盘屏障是营养物质以及某些药物、病毒、激素等从母体进入胎儿的必经之路, 而滋养层细胞是胎盘屏障的第-道防线. 本研究用HCV RNA阳性血清感染体外培养的人胎盘滋养层细胞, 并运用透射电镜及激光共聚焦技术观察滋养层细胞感染HCV后的特性, 为进一步研究HCV的母婴传播机制提供形态学基础.
Percoll细胞分离液为美国Pharmacia公司产品(No: 05-59-16-001A), 免疫组织化学染色用ABC试剂盒购自华美生物工程公司, 所用细胞角蛋白(No: C8541)及波形蛋白抗体(No: MU074-UC)购自宝泰克生物工程公司. HCV RNA荧光定量检测试剂盒购自美国Acegene公司.
取剖腹产术后的无菌胎盘组织, 将绒毛组织剪碎至1-3 mm3, 以0.25%胰蛋白酶液及20 u/mL DNaseⅠ于37℃恒温摇床内消化30 min, 消化产物经100目不锈钢筛网过滤后, 滤液加入预先加有5 mL小牛血清的50 mL离心管内, 1 000 rpm/min离心10 min, 弃上清, DMEM培养液重悬沉淀.
用9.0% NaCl与Percoll以1: 9混合, 以达到生理性渗透压, 再以0.9% NaCl稀释为45%和35%两个浓度, 按浓度从大到小将两个不同密度的Percoll溶液逐层缓慢加入10 mL离心管内, 每个浓度3 mL.
将收集的细胞悬液2 mL缓慢加于Percoll分离液上层, 以2 500 rpm ·min-1离心20 min, 用吸管小心吸取云雾状的中层置于-50 mL离心管内, DMEM稀释4倍, 1 000 rpm/min离心10 min, 弃上清, 以含20%胎牛血清DMEM培养液重悬沉淀, 将细胞悬液浓度调整至5-6×109·L-1, 置于培养瓶或预先置有盖玻片的六孔培养板内37℃, 5%CO2孵箱内培养.
细胞爬片固定后, 用ABC法进行细胞角蛋白、波形蛋白染色, 操作步骤按说明书进行. 胎盘组织切片脱蜡至水后用ABC法进行细胞角蛋白、波形蛋白染色.
HCV感染血清选择抗HCV阳性, HCV RNA6.1×107 copies. L-1的血清, 经血清学检查甲、乙、丁、戊肝炎病毒标志物均为阴性, 同时用正常人血清作为阴性对照.
以含20%丙型肝炎患者血清(HCV RNA检测阳性)的DMEM培养液感染体外培养24 h后的滋养层细胞. 同时分别含20%正常人血清的DMEM培养液感染体外培养24 h后的滋养层细胞作为阴性对照, 37℃, 5% CO2孵箱内培养24 h后倒置显微镜下观察, 并进行第1次换液, 弃去感染血清, PBS充分洗涤5次后加入新鲜培养液, 留取最后1次洗液待检, 以后每2 d换液1次, 留取培养上清待检.
为保证试验可靠性, 分别采用逆转录聚合酶链反应(RT-PCR)及扩增敏感试验(amplisensor assay)对培养上清中HCV RNA进行定性及定量检测, 具体步骤按操作说明书进行.
培养上清的HCV RNA定性检测简要步骤如下: 提取HCV RNA, 加入逆转录反应体系, 37℃, 30 min: 预变性94℃, 300 s, 第1次PCR循环, 94℃ 45 s, 50℃ 45 s, 72℃ 45 s循环35次, 终延伸72℃, 300 s; 取2 mL第1次循环后的产物加入第2次PCR反应缓冲液23 mL; 预变性94℃, 300 s, 第2次PCR循环, 94℃ 45 s, 55℃ 45 s, 72℃ 45 s循环35次, 终延伸72℃, 300 s; 取扩增产物15 mL加样于含溴化乙锭的2%琼脂糖凝胶, 于1×TAE缓冲液中在60 v电压下电泳15-20 min. 根据试剂盒说明, 紫外灯下观察225 bp处有荧光者为阳性.
培养上清的HCV RNA定量检测简要步骤如下: 提取HCV RNA, 加入逆转录反应体系, 37℃, 45 min: 预变性90℃, 2 min, PCR循环, 95℃ 25 s, 60℃ 35 s, 72℃ 45 s循环20次, 终延伸72℃, 30 sec, 10℃保温1 h; 计算机读取数值, >104者为阳性.
细胞培养40 d后以0.25%胰蛋白酶分别消化感染组及对照组贴壁生长的细胞, PBS洗涤1次, 2 000 rpm. min-1离心20 min, 4%戊二醛固定过夜, 送本校电镜室.
本方法分离纯化后的中层细胞在倒置显微镜下观察以单个核细胞为主, 培养3 h后开始贴壁, 18 h后基本全部贴壁, 贴壁细胞多呈上皮细胞样生长, 形态多样, 个别细胞呈纤维细胞样. 24 h后开始出现多个核细胞, 并随时间推移逐渐增大, 成片生长. HCV感染后, 感染组及对照组细胞光镜下观察未见明显不同.
细胞爬片染色阳性信号为棕黄色颗粒, 结果显示, 90%以上细胞的细胞角蛋白染色阳性, 阳性信号定位于细胞质, 而波形蛋白染色仅个别细胞可见阳性信号.
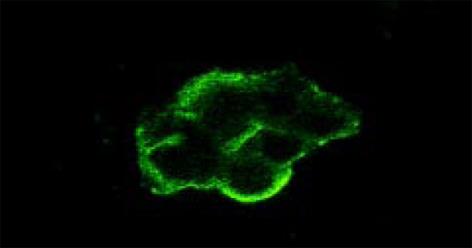
HCV感染后的滋养层细胞抗-HCV NS5染色阳性, 阳性信号为黄绿色或黄色荧光, 主要定位于细胞核周. 正常对照标本的滋养层细胞抗-HCV NS5染色未见黄绿色或黄色荧光, 因伊文蓝衬染而呈现红色.
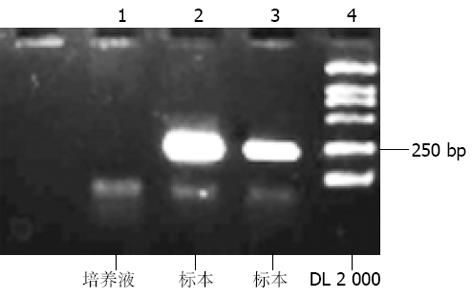
对收集的感染前、感染血清、感染后洗液、感染后2-16 d培养上清共11份标本进行HCV RNA定性(图2)定量检测, 结果如下(见表1).
| 定量检测(单位: copies/mL) | 定性检测 | |||
| 感染组 | 对照组 | 感染组 | 对照组 | |
| 感染前 | <104 | <104 | - | - |
| 感染血清 | 6.1×104 | <104 | - | - |
| 感染后洗液 | <104 | <104 | - | - |
| 感染后2 d | 1.4×104 | <104 | - | - |
| 感染后4 d | <104 | <104 | - | - |
| 感染后6 d | 9.7×104 | <104 | - | - |
| 感染后8 d | <104 | <104 | - | - |
| 感染后10 d | 2.5×105 | <104 | + | - |
| 感染后12 d | 1.5×104 | <104 | - | - |
| 感染后14 d | 6.0×105 | <104 | - | - |
| 感染后16 d | 4.0×105 | <104 | + | - |
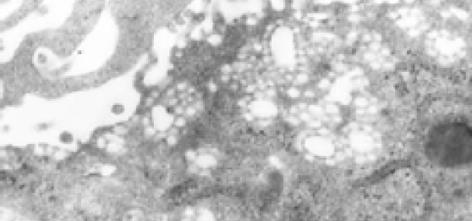
透射电镜下未感染HCV的滋养层细胞直径16 mm左右, 表面微绒毛发达, 可见较多被膜小凹, 胞质内可见较多包膜小泡及丰富的微丝, 脂滴较多. 感染HCV后的滋养层细胞超微结构发生了明显的改变主要表现如下:
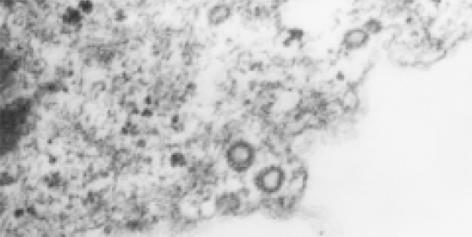
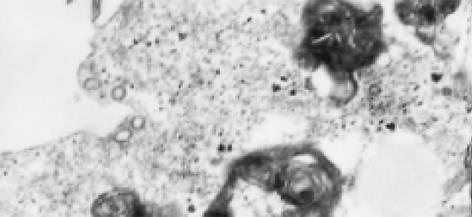
2.6.1 病毒样颗粒: 在HCV感染后的滋养层细胞内观察到许多病毒颗粒样结构(图3), 位于胞质内或空泡状结构内, 圆球形或椭圆形, 有明确的外膜, 核衣壳样结构外表面为细长的钉状突起, 核心直径50 nm左右. 在细胞膜上发现类似病毒内吞和芽生的形态.
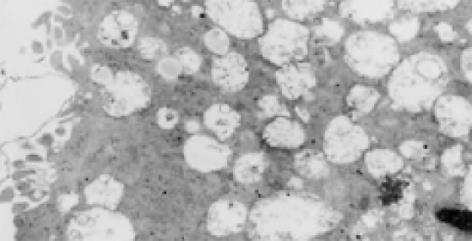
2.6.2 溶酶体大量增生: 感染了HCV的滋养层细胞内可见大量的溶酶体增生, 增生的溶酶体有两种形态: (1)空泡状溶酶体: 细胞电子密度降低, 胞质被大量增生的溶酶体所占据, 增生的溶酶体内可见许多更小的空泡状结构(图4); (2)髓鞘状溶酶体: 细胞电子密度较高, 胞质内可见大量电子密度较高的溶酶体, 形成髓鞘状(图5).
2.6.3 空泡状结构: HCV感染的滋养层细胞内可以看到许多聚集存在的花环状排列的空泡结构, 并有大量小囊泡结构, 其内亦可见到类似病毒核衣壳样小空泡(图6).
2.6.4 粗面内质网增生: HCV感染的滋养层细胞内可见粗面内质网增生, 粗面内质网内可见细丝状物和小泡状结构, 小泡膜似与粗面内质网内膜相连.
2.6.5 脂滴减少: 正常滋养层细胞内可见大量脂滴, 而感染了HCV的细胞内脂滴明显减少.
胎盘屏障是营养物质以及某些药物、病原体、激素等从母体进入胎儿的必经之路, 合体滋养层细胞还具有重要的内分泌功能, 体外分离培养滋养层细胞是研究其功能及母体与胎儿之间物质交换的具体分子机制的细胞学基础, 本研究在Kaldor et al[34]建立的Percoll不连续密度梯度分离纯化方法基础上作如下改进.
将分离细胞的密度梯度简化为两层: Kliman et al用14个不连续Percoll分离密度梯度进行分离, 并确定滋养层细胞在Percoll中所处的密度值为1 048-1 062 g/L, 而Nie et al[35]用连续密度梯度观察其密度值为1 053-1 060 g/L, 以此为依据并根据美国Pharmacia公司提供的Percoll使用说明书中不同稀释度的Percoll在0.15M (0.9%)盐溶液中相应的密度值曲线, 将分离梯度简化为两层, 即35%(密度值为1 043 g/L)与45%(密度值为1 062 g/L), 以使所要分离的滋养层细胞处于二层之间, 这样既节约了实验试剂, 又简化了操作步骤.
许多学者研究证实, 在胎盘绒毛组织中细胞角蛋白染色阳性的仅细胞滋养层细胞及合体滋养层细胞[34-38]. 本研究培养细胞爬片免疫组化染色结果显示, 细胞角蛋白染色阳性者占90%以上, 证明改进后的分离方法同样可以得到满意的分离效果.
目前, HCV感染靶细胞的方式主要有血清直接感染法、脂质体介导法、电穿孔法及真核细胞表达载体携带法等. 直接感染法是HCV感染细胞的主要方法, 即把丙型肝炎患者血清直接加到培养细胞中进行感染, 37℃温育不同时间后, 更换新鲜培养液, 在不同时相检测细胞及其培养上清中的HCV RNA. RT-PCR用于直接检测培养上清及细胞中HCV正链及负链RNA, 可直观反映HCV在感染细胞中的复制情况. 该方法简便, 特异性好, 但培养液中残留的HCV感染物可能会出现假阳性结果.
本研究参照国外学者[39-42]用HCV感染体外培养细胞的方法, 对滋养层细胞进行感染试验. 经定性及定量RT-PCR双重检测, 证明了实验的可靠性. 结果表明, 感染前及感染后洗液及对照组中HCV RNA检测均为阴性, 排除了标本来源及HCV感染试验时培养液中残留HCV感染物污染的可能. 在感染后16 d的培养上清中间断检测到了HCV RNA. HCV RNA定性检测结果为阳性标本HCV RNA定量检测>105.证实HCV可以感染胎盘滋养层细胞, 但这种感染是否为-过性感染, HCV能否在滋养层细胞中复制尚有待进一步研究.
采用电子显微镜技术直接观察转染细胞内或培养上清内的HCV颗粒及细胞的超微结构改变, 这也是判定HCV转染是否成功的一个可靠标准. 1994年Kaitol et al[43]首次用特异性的抗假定HCV包膜蛋白的多克隆抗体及单克隆抗体, 对含高效价HCV RNA而HBsAg、抗-HTLV1和HIV均阴性的血浆标本经反复超速离心并经连续蔗糖密度梯度分离, 在密度1 140-1 160 g/L组分中见到55-65 nm球形病毒样颗粒, 有棘状突起, 形态特性与其他黄病毒相似. 进行间接免疫电镜鉴定, 发现病毒外膜为胶体金颗粒特异标记, 证明所见确为HCV颗粒. 关于病毒颗粒的大小, 论文作者描述为直径55-65 nm, 但陈良标et al[39]根据论文照片提供的标尺测量, 病毒的实际大小是: 含包膜的完整病毒直径110-130 nm, 内含直径55-65 nm圆球形核心或核壳体. 核壳体表面6 nm长的细钉状突起, 可能是病毒核壳体的子粒结构. 病毒包膜与核心之间间距25-35 nm, 类似疱疹病毒形态. 病毒包膜不稳定, 纯化过程中易破裂丢失, 故不少报告HCV直径55-65 nm[44-50], 实际可能是指病毒的核心结构.
本研究中采用透射电镜观察HCV感染后滋养层细胞的超微结构改变, 发现正常成熟细胞滋养层细胞有丰富的表面微绒毛, 被膜小凹, 细胞内包膜小泡、脂滴、粗面内质网、微丝等结构, 这些结构有效地增加了母体血与胎儿血的接触面积, 并有利于其完成合成蛋白、激素及物质转运功能. HCV感染后滋养层细胞超微结构发生了明显改变, 主要表现为溶酶体大量增生, 粗面内质网增生, 脂滴减少, 胞质内及胞质小囊泡内存在大量聚集分布的空泡状结构, 其形态类似于病毒核衣壳. 溶酶体的增生为细胞受到感染后的表现, 但为何有些细胞出现空泡状溶酶体, 而有些则为髓鞘样溶酶体尚有待研究. 有意义的是在感染后的细胞胞质内存在许多病毒颗粒样结构, 核心直径约为50 nm左右, 有明确的外膜和棘突, 完整病毒颗粒直径约100 nm左右. 这些形态特征与其他黄病毒科病毒相似, 黄病毒科病毒复制过程中-般伴随特征性的粗面和滑面内质网增生, 形成众多微泡或板层状膜结构. 并且感染后的滋养层细胞超微结构改变与目前研究的HCV感染人外周血淋巴细胞、人胎肝细胞等[38-42]的改变相似.
HCV NS5为HCV非结构蛋白之一, 目前对其功能研究得较为明确, HCV NS5主要表达RDRP, 参与HCV的复制, 并具有外周核定位功能, 提示HCV的复制位于核周围的内质网膜上. 用特异性抗-HCVMcAb NS5 , 采用间接免疫荧光法直接检测感染细胞内特异性抗原的表达情况, 并用激光共聚焦方法观察发现, HCV NS5 主要在核周表达.
这些改变为HCV感染滋养层细胞提供了形态学依据, 提示HCV可以感染滋养层细胞, 并有可能因此导致HCV的母婴传播, 但HCV感染后滋养层细胞中的病毒样颗粒尚有待免疫电镜、原位杂交等方法的证实, HCV能否在滋养层细胞中复制并产生有感染性的病毒颗粒尚在进一步研究中.
编辑: N/A
| 1. | Resti M. Mother-to-infant transmission of hepatitis C virus. Ital J Gastroenterol Hepatol. 1999;31:489-493. [PubMed] |
| 2. | Cheng YQ, Nie QH, Zhou YX. Study on mother-to-infant transmission mechanisms of hepatitis C virus. Shijie Huaren Xiaohua Zazhi. 2002;10:445-447. [DOI] |
| 3. | Conte D, Colucci A, Minola E, Fraquelli M, Prati D. Clinical course of pregnant women with chronic hepatitis C virus infection and risk of mother-to-child hepatitis C virus transmission. Dig Liver Dis. 2001;33:366-371. [PubMed] [DOI] |
| 4. | Healy CM, Cafferkey MT, Conroy A, Dooley S, Hall WW, Beckett M, Clarke TA, White MJ, Gorman WA, Butler KM. Outcome of infants born to hepatitis C infected women. Ir J Med Sci. 2001;170:103-6; discussion 92-3. [PubMed] [DOI] |
| 5. | Rubio Quevedo C, Holgado Carballo MA, García Suárez A, Martín de Lara IM, Moltó Ripoll L. [Vertical transmission of hepatitis C virus]. An Esp Pediatr. 2001;54:27-31. [PubMed] [DOI] |
| 6. | Kaldor JM, Dore GJ, Correll PK. Public health challenges in hepatitis C virus infection. J Gastroenterol Hepatol. 2000;15 Suppl:E83-E90. [PubMed] [DOI] |
| 7. | Polatti F, Viazzo F, Colleoni R, Belloni C, Zara F. Vertical transmission of hepatitis C virus and follow-up of newborns from infected mothers. Minerva Ginecol. 2000;52:59-62. [PubMed] |
| 8. | Resti M, Bortolotti F, Azzari C, Giacchino R, Zancan L, Gussetti N, Vierucci A. Transmission of hepatitis C virus from infected mother to offspring during subsequent pregnancies. J Pediatr Gastroenterol Nutr. 2000;30:491-493. [PubMed] [DOI] |
| 9. | Okamoto M, Nagata I, Murakami J, Kaji S, Iitsuka T, Hoshika T, Matsuda R, Tazawa Y, Shiraki K, Hino S. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514. [PubMed] [DOI] |
| 10. | Ceci O, Margiotta M, Marello F, Francavilla R, Loizzi P, Francavilla A, Mautone A, Impedovo L, Ierardi E, Mastroianni M. Vertical transmission of hepatitis C virus in a cohort of 2,447 HIV-seronegative pregnant women: a 24-month prospective study. J Pediatr Gastroenterol Nutr. 2001;33:570-575. [PubMed] [DOI] |
| 11. | Tajiri H, Miyoshi Y, Funada S, Etani Y, Abe J, Onodera T, Goto M, Funato M, Ida S, Noda C. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14. [PubMed] [DOI] |
| 12. | Gibb DM, Goodall RL, Dunn DT, Healy M, Neave P, Cafferkey M, Butler K. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907. [PubMed] [DOI] |
| 13. | Paternoster DM, Santarossa C, Grella P, Palù G, Baldo V, Boccagni P, Floreani A. Viral load in HCV RNA-positive pregnant women. Am J Gastroenterol. 2001;96:2751-2754. [PubMed] [DOI] |
| 14. | Wejstål R, Månson AS, Widell A, Norkrans G. Perinatal transmission of hepatitis G virus (GB virus type C) and hepatitis C virus infections--a comparison. Clin Infect Dis. 1999;28:816-821. [PubMed] [DOI] |
| 15. | Kudo T, Yanase Y, Ohshiro M, Yamamoto M, Morita M, Shibata M, Morishima T. Analysis of mother-to-infant transmission of hepatitis C virus: quasispecies nature and buoyant densities of maternal virus populations. J Med Virol. 1997;51:225-230. [PubMed] [DOI] |
| 16. | Giovannetti A, Mazzetta F, Coviello R, Casadei AM, Sartorerri MR, Marziali M, Pierdominici M, Kuonen D, Pesce AM, Fiorilli M. T-Cell immune activation in children with vertically transmitted hepatitis C virus infection. Viral Immunol. 2001;14:169-179. [PubMed] [DOI] |
| 17. | Mast EE, Alter MJ, Margolis HS. Strategies to prevent and control hepatitis B and C virus infections: a global perspective. Vaccine. 1999;17:1730-1733. [PubMed] [DOI] |
| 18. | Ou-Yang P, Hwang LH, Tao MH, Chiang BL, Chen DS. Co-delivery of GM-CSF gene enhances the immune responses of hepatitis C viral core protein-expressing DNA vaccine: role of dendritic cells. J Med Virol. 2002;66:320-328. [PubMed] [DOI] |
| 19. | Liu ZX, Nishida H, He JW, Lai MM, Feng N, Dennert G. Hepatitis C virus genotype 1b core protein does not exert immunomodulatory effects on virus-induced cellular immunity. J Virol. 2002;76:990-997. [PubMed] [DOI] |
| 20. | Liu J, Zhu L, Zhang X, Lu M, Kong Y, Wang Y, Li G. Expression, purification, immunological characterization and application of Escherichia coli-derived hepatitis C virus E2 proteins. Biotechnol Appl Biochem. 2001;34:109-119. [PubMed] [DOI] |
| 21. | Roccasecca R, Folgori A, Ercole BB, Puntoriero G, Lahm A, Zucchelli S, Tafi R, Pezzanera M, Galfre G, Tramontano A. Mimotopes of the hyper variable region 1 of the hepatitis C virus induce cross-reactive antibodies directed against discontinuous epitopes. Mol Immunol. 2001;38:485-492. [PubMed] [DOI] |
| 22. | Hunziker IP, Zurbriggen R, Glueck R, Engler OB, Reichen J, Dai WJ, Pichler WJ, Cerny A. Perspectives: towards a peptide-based vaccine against hepatitis C virus. Mol Immunol. 2001;38:475-484. [PubMed] [DOI] |
| 23. | Moriya O, Matsui M, Osorio M, Miyazawa H, Rice CM, Feinstone SM, Leppla SH, Keith JM, Akatsuka T. Induction of hepatitis C virus-specific cytotoxic T lymphocytes in mice by immunization with dendritic cells treated with an anthrax toxin fusion protein. Vaccine. 2001;20:789-796. [PubMed] [DOI] |
| 24. | Fournillier A, Wychowski C, Boucreux D, Baumert TF, Meunier JC, Jacobs D, Muguet S, Depla E, Inchauspé G. Induction of hepatitis C virus E1 envelope protein-specific immune response can be enhanced by mutation of N-glycosylation sites. J Virol. 2001;75:12088-12097. [PubMed] [DOI] |
| 25. | Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. Cross-reactivity between hepatitis C virus and Influenza A virus determinant-specific cytotoxic T cells. J Virol. 2001;75:11392-11400. [PubMed] [DOI] |
| 26. | Begemann M, Policar M. Pneumococcal vaccine failure in an HIV-infected patient with fatal pneumococcal sepsis and HCV-related cirrhosis. Mt Sinai J Med. 2001;68:396-399. [PubMed] |
| 27. | Prince AM, Shata MT. Immunoprophylaxis of hepatitis C virus infection. Clin Liver Dis. 2001;5:1091-1103. [PubMed] [DOI] |
| 28. | Chlabicz S, Grzeszczuk A. Prevalence of hepatitis B markers in patients with hepatitis C infection in north-eastern Poland: risk factors and vaccine use. Eur J Epidemiol. 2001;17:267-270. [PubMed] [DOI] |
| 29. | Wedemeyer H, Gagneten S, Davis A, Bartenschlager R, Feinstone S, Rehermann B. Oral immunization with HCV-NS3-transformed Salmonella: induction of HCV-specific CTL in a transgenic mouse model. Gastroenterology. 2001;121:1158-1166. [PubMed] [DOI] |
| 30. | Aslam M, Aslam J. Seroprevalence of the antibody to hepatitis C in select groups in the Punjab region of Pakistan. J Clin Gastroenterol. 2001;33:407-411. [PubMed] [DOI] |
| 31. | Merola M, Brazzoli M, Cocchiarella F, Heile JM, Helenius A, Weiner AJ, Houghton M, Abrignani S. Folding of hepatitis C virus E1 glycoprotein in a cell-free system. J Virol. 2001;75:11205-11217. [PubMed] [DOI] |
| 32. | Nie Q, Li M. [Current status and strategy of the research on hepatitis C vaccine]. Zhonghua Liu Xing Bing Xue Za Zhi. 1998;19:47-49. [PubMed] |
| 33. | Mele A, Spada E, Sagliocca L, Ragni P, Tosti ME, Gallo G, Moiraghi A, Balocchini E, Sangalli M, Lopalco PL. Risk of parenterally transmitted hepatitis following exposure to surgery or other invasive procedures: results from the hepatitis surveillance system in Italy. J Hepatol. 2001;35:284-289. [PubMed] [DOI] |
| 34. | Kaldor JM, Dore GJ, Correll PK. Public health challenges in hepatitis C virus infection. J Gastroenterol Hepatol. 2000;15:83-90. |
| 35. | Nie QH. Mother-to-infant infection of hepatitis C virus. Shiyong Ganzangbing Zazhi. 2000;5:52-53. |
| 36. | Polatti F, Viazzo F, Colleoni R, Belloni C, Zara F. Vertical transmission of hepatitis C virus and follow-up of newborns from infected mothers. Minerva Ginecol. 2000;52:59-62. |
| 37. | Resti M, Bortolotti F, Azzari C, Giacchino R, Zancan L, Gussetti N, Vierucci A. Transmission of hepatitis C virus from infected mother to offspring during subsequent pregnancies. J Pediatr Gastroenterol Nutr. 2000;30:491-493. |
| 38. | Kaito M, Watanabe S, Tsukiyama-Kohara K, Yamaguchi K, Kobayashi Y, Konishi M, Yokoi M, Ishida S, Suzuki S, Kohara M. Hepatitis C virus particle detected by immunoelectron microscopic study. J Gen Virol. 1994;75:1755-1760. |
| 39. | Chen LB. Application of electron microscope on the diagnosis and study of viral hepatitis. Dianzi Xianwei Xuebao. 1997;16:574-587. |
| 40. | Choo SH, So HS, Cho JM, Ryu WS. Association of hepatitis C virus particles with immunoglobulin: a mechanism for persistent infection. J Gen Virol. 1995;76:2337-2341. [PubMed] [DOI] |
| 41. | Watson JP, Bevitt DJ, Spickett GP, Toms GL, Bassendine MF. Hepatitis C virus density heterogeneity and viral titre in acute and chronic infection: a comparison of immunodeficient and immunocompetent patients. J Hepatol. 1996;25:599-607. [PubMed] [DOI] |
| 42. | Trestard A, Bacq Y, Buzelay L, Dubois F, Barin F, Goudeau A, Roingeard P. Ultrastructural and physicochemical characterization of the hepatitis C virus recovered from the serum of an agammaglobulinemic patient. Arch Virol. 1998;143:2241-2245. [PubMed] [DOI] |
| 43. | Kaito M, Watanabe S, Tsukiyama-Kohara K, Yamaguchi K, Kobayashi Y, Konishi M, Yokoi M, Ishida S, Suzuki S, Kohara M. Hepatitis C virus particle detected by immunoelectron microscopic study. J Gen Virol. 1994;75:1755-1760. [PubMed] [DOI] |
| 44. | De Vos R, Vanstapel MJ, Desmyter J, De Wolf-Peeters C, De Groote G, Colaert J, Mortelmans J, De Groote J, Fevery J, Desmet V. Are nuclear particles specific for non-A, non-B hepatitis? Hepatology. 1983;3:532-544. [PubMed] [DOI] |
| 45. | Iwarson S, Schaff Z, Seto B, Norkrans G, Gerety RJ. Retrovirus-like particles in hepatocytes of patients with transfusion-acquired non-A, non-B hepatitis. J Med Virol. 1985;16:37-45. [PubMed] [DOI] |
| 46. | Feinstone SM, Mihalik KB, Kamimura T, Alter HJ, London WT, Purcell RH. Inactivation of hepatitis B virus and non-A, non-B hepatitis by chloroform. Infect Immun. 1983;41:816-821. [PubMed] |
| 47. | Bradley DW, McCaustland KA, Cook EH, Schable CA, Ebert JW, Maynard JE. Posttransfusion non-A, non-B hepatitis in chimpanzees. Physicochemical evidence that the tubule-forming agent is a small, enveloped virus. Gastroenterology. 1985;88:773-779. [PubMed] [DOI] |
| 48. | An P, Chen L, Tian H, Chen P, Li L, Liu C. [The significance of detection of hepatitis C virus RNA in peripheral blood mononuclear cells of patients with chronic hepatitis C]. Zhonghua Nei Ke Za Zhi. 1999;38:737-739. [PubMed] |
| 49. | Chen LB, Chen PL, Liu CY. Identification and visualzation of virus-like particles in peripheral blood mononuclear cells (PBMCs) of patients with hepatitis C. Zhongguo Yixue Yingxiangxue Zazhi. 1999;7:110-111. |