修回日期: 2002-11-01
接受日期: 2002-11-16
在线出版日期: 2003-02-15
目的: 探讨预先转染丙型肝炎病毒(HCV)核酶(Rz213)的人肝癌细胞对HCV再转染的抑制作用.
方法: 应用从前构建的HCV锤头结构Rz(Rz213), 通过脂质体介导的基因转染方法, 转染人肝癌细胞(HHCC), 经G418筛选转染Rz213的基因克隆, 应用luc报告基因的表达活性, 观察该细胞克隆对靶基因(pCMVNCR luc)转染的抑制作用.
结果: G418筛选能使 Rz213在转染细胞内有效表达Rz RNA, 转染Rz的多克隆细胞能有效地阻断靶基因的再转染.
结论: 我们构建的Rz213真核表达载体能在HHCC细胞内有效表达, 预先转染发挥细胞内免疫作用, 可防止HCV感染.
引文著录: 贾战生, 陈琳, 郝春秋, 冯志华, 李谨革, 王九平, 曹义战, 周永兴. 丙型肝炎病毒锤头结构核酶的细胞内免疫. 世界华人消化杂志 2003; 11(2): 148-150
Revised: November 1, 2002
Accepted: November 16, 2002
Published online: February 15, 2003
AIM: To evaluate the effect of hammerhead ribozyme 213 (Rz 213) against hepatitis C virus (HCV) infection.
METHODS: Rz213 cleaving 5'oncoding region (5'CR) of HCV was beforehand transfected in a human hepatic carcinoma cell (HHCC) line and selected for G418 resistance. Cells stably expressing Rz213 were retransfected with pCMVNCRluc containing 5扤CR-luc fusion genes by lipofectAMINE; luciferase activity in lysate of transfactant was measured in scintillation counter.
RESULTS: HHCC cells stably expressing Rz213 exhibited significant resistance to retransfection of targeting gene.
CONCLUSION: Stably transfected cells with Rz213 were selected and expressed in HHCC, and thus exerted the intracellular immunity against infection of HCV.
- Citation: Jia ZS, Chen L, Hao CQ, Feng ZH, Li JG, Wang JP, Cao YZ, Zhou YX. Intracellular immunization by hammerhead ribozyme against HCV. Shijie Huaren Xiaohua Zazhi 2003; 11(2): 148-150
- URL: https://www.wjgnet.com/1009-3079/full/v11/i2/148.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i2.148
丙型肝炎病毒(HCV)非编码区(5'NCR)和核心(C)区高度保守, 具有重要的生物学功能, 是基因治疗选择的理想靶序列[1-5]. 锤头结构核酶(hammerhead Rz)分子较小, 结构简单, 易于体外设计合成, 已广泛用于抗病毒基因治疗研究[6-13]. 应用Rz基因转染技术作为细胞内免疫的工具, 由Baltimore at al首次提出, 并应用于艾滋病毒(HIV)的防治研究. Heusch at al 应用发夹(hairpin)结构核酶作为细胞内免疫分子, 预先转染人CEM/1细胞证明, 该细胞可以有效抵抗HIV病毒感染. 我们从前的研究结果表明, 以HCV 5'NCR为靶序列设计的锤头结构Rz, 可在细胞内有效地抑制靶基因的表达[10,14,15]. 本研究探讨了抗HCV 5'NCR核酶预先转染靶细胞的预防作用.
人肝癌细胞株(human hepatic carcinoma cell, HHCC)由中科院上海细胞所细胞库引种, 本校863研究室保存. 核酶真核表达载体pcEM-Rz213 (Rz213)由本室自行设计构建, 并经测序体外切割证明[14]. 靶基因pCMVNCRluc含有HCV 5'NCR和C区66 nt, 并与luc基因融合, 由德国的Alt教授惠赠[1]. 转染方法按试剂盒说明操作(Lipofectamine和G418为Gibco产品).
通过显微镜观察Rz转染对细胞生长特性的影响, 同时收集对数生长期细胞, 用胰酶消化, 尽可能消化成单个细胞悬液, 75 mL/L乙醇固定, 流式细胞仪检测细胞周期. 细胞转染Rz213 48 h后, 传代细胞(1:5)于6孔和24孔培养板内, 换用350 mg/L的G418完全培养液, 筛选转染细胞, 每3 d换培养液1次. 不同时间收集细胞, 制备裂解液. 留-部分做克隆筛选, 直至细胞克隆出现. 荧光素酶(Luc)活性检测按试剂盒说明操作(luciferase assay system 为 promega产品). 取20 μL 细胞裂解液, 加入1.5 mL Eppendorf管, 加100 uL luc测试液, 混匀; 尽快用液体闪烁计数仪计1 min的cpm值[15]. 同时用Braford法测定20 μL裂解液中的蛋白含量, 最后核算成每 mg蛋白所含的计数值.
统计学处理 数据均以均值加减标准差表示. 两组间比较采用t检验, 多组间比较采用方差分析.
将pcEM-Rz213转染的HHCC细胞多克隆(20 d), 传代取对数生长期细胞, 作细胞周期检测, 同时用转染空载体的HHCC细胞对照. 结果表明, 两组之间无显著意义, 说明Rz和载体在短期内对细胞的增生周期可能无影响.
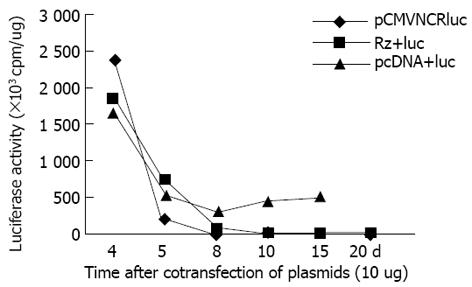
pCMVNCRluc为瞬时表达载体, 缺乏新霉素基因, 与pcDNA3共转染, 经G418筛选可明显延长luc的表达. 然而, 该载体与Rz213共转染, G418筛选提高了Rz基因的表达, luc活性并没有延长, 说明Rz基因在细胞内抑制了luc的表达. 靶基因组、Rz+靶基因组和对照组(空载体+靶基因)luc活性均随时间下降, 但在第5天时各组之间就有显著差异, 对照组最高, Rz组最低. 8 d后见Rz组luc活性明显低于对照组. 随G418筛选时间延长(最长20 d), 仅对照组可检测到luc活性, 但cpm值均在5 000以下, 远比前72 h低(图1).
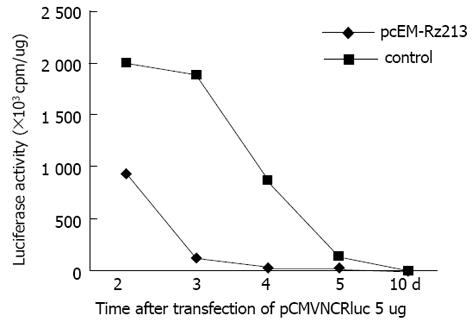
Rz213与pCMVNCRluc靶基因共转染细胞, Rz213可有效地抑制luc的活性. 为了证明Rz对细胞的保护作用, 预先将pcEM-Rz213转染HHCC细胞, 经G418筛选抗性多克隆细胞. 以此为靶再用pCMVNCRluc 转染. 结果表明, 预先转染pcEM-Rz213的HHCC细胞可有效地阻断再转染靶基因的表达(图2).
细胞内免疫包括细胞内抗体、DNA免疫、核酶和细胞因子基因转染等[16-21], 是抗病毒基因治疗的重要手段之一. 其主要步骤: 将目的基因转入靶细胞, 使靶细胞稳定表达某种抗病毒分子, 通过细胞内分子的作用, 有效地抑制病毒基因的表达和复制, 因此转入该基因的细胞能有效地抵抗病毒的攻击, 达到细胞内免疫的目的. Yu at al应用逆转录病毒为载体, 将抗HIV-1病毒的核酶基因转入胎儿脐血CD34+干细胞, 结果发现随细胞分化增生, 核酶基因仍有效表达, 转染目的基因的细胞可抵抗HIV-1病毒的感染. 最近, Fraisier et al[22]应用抗tat核酶和抗TAR的RNA诱饵联合细胞内免疫, 并在靶基因的3'端添加b-球蛋白的3'非编码序列, 增加了靶基因的稳定性, 有效地提高了抗HIV效果.
HCV感染者接受肝移植后, 发生移植肝再感染HCV是导致肝脏移植失败的主要原因[23]. 应用核酶技术, 采用HIV细胞内免疫的思路, 有可能防止移植肝再感染HCV. HCV核酶作为基因治疗的手段之一, 已经多位学者证明, 可有效地抑制病毒基因的表达[11,12,22-24]. 我们在研究了抗HCV Rz在细胞内抑制病毒基因表达的基础上, 探讨了该Rz预先转染肝细胞的细胞内免疫效应. 结果表明, 抗HCV 5'NCR Rz213预先转染的HHCC细胞, 可有效地表达Rz RNA分子, 经G418筛选的抗性多克隆细胞能够有效地抵抗靶基因的再转染. 提示将抗HCV Rz预先导入细胞, 有可能象HCV"疫苗"免疫-样, 防止HCV感染, 这对于慢性肝病晚期接受肝移殖的患者可能是一种新治疗希望, 预先将抗HCV Rz导入移殖的肝细胞, 以防止移殖肝再感染HCV.
基因治疗能否应用于临床, -方面取决于基因治疗的有效性, 另一方面由其对机体的副作用决定[25-27]. 抗HCV Rz载体介导的内源性Rz在细胞内有效表达是Rz清除病毒的基础. 借鉴体外切割实验的数据, Rz在细胞内要达到完全清除病毒, 其分子数必需是靶基因的100倍以上, 并要求Rz能到达所有HCV感染的肝细胞和非肝细胞[4,24]. 我们选择pcDNA3作为真核载体是-高效真核表达载体, 结果表明, 经G418抗性筛选可以提高Rz的表达. 细胞生长特性和细胞周期的研究表明, 该载体和Rz基因近期对靶细胞无毒副作用.
编辑: N/A
| 1. | Alt M, Eisenhardt S, Serwe M, Renz R, Engels JW, Caselmann WH. Comparative inhibitory potential of differently modified antisense oligodeoxynucleotides on hepatitis C virus translation. Eur J Clin Invest. 1999;29:868-876. [PubMed] [DOI] |
| 2. | Pybus OG, Charleston MA, Gupta S, Rambaut A, Holmes EC, Harvey PH. The epidemic behavior of the hepatitis C virus. Science. 2001;292:2323-2325. [PubMed] [DOI] |
| 3. | Rosenberg S. Recent advances in the molecular biology of hepatitis C virus. J Mol Biol. 2001;313:451-464. [PubMed] [DOI] |
| 4. | Drazan KE. Molecular biology of hepatitis C infection. Liver Transpl. 2000;6:396-406. [PubMed] [DOI] |
| 5. | Cornberg M, Wedemeyer H, Manns MP. Hepatitis C: therapeutic perspectives. Forum (Genova). 2001;11:154-162. [PubMed] |
| 6. | Schmitz V, Qian C, Ruiz J, Sangro B, Melero I, Mazzolini G, Narvaiza I, Prieto J. Gene therapy for liver diseases: recent strategies for treatment of viral hepatitis and liver malignancies. Gut. 2002;50:130-135. [PubMed] [DOI] |
| 7. | Ni YH. In vivo hepatic gene therapy. Acta Paediatr Taiwan. 2001;42:191-200. [PubMed] |
| 8. | Feng Y, Kong YY, Wang Y, Qi GR. Inhibition of hepatitis B virus by hammerhead ribozyme targeted to the poly(A) signal sequence in cultured cells. Biol Chem. 2001;382:655-660. [PubMed] [DOI] |
| 9. | Macejak DG, Jensen KL, Jamison SF, Domenico K, Roberts EC, Chaudhary N, von Carlowitz I, Bellon L, Tong MJ, Conrad A. Inhibition of hepatitis C virus (HCV)-RNA-dependent translation and replication of a chimeric HCV poliovirus using synthetic stabilized ribozymes. Hepatology. 2000;31:769-776. [PubMed] [DOI] |
| 10. | Jia ZS, Zhou YX, Lian JQ, Feng ZH, Li GY, Zhang WB. Computer design of the hammerhead ribozyme against hepatitis C virus. Shijie Huaren Xiaohua Zazhi. 1999;7:300-302. |
| 11. | Wen SJ, Xiang KJ, Huang ZH, Zhou R, Qi XZ. Construction of HBV-specific ribozyme and its recombinant with HDV and their cleavage activity in vitro. World J Gastroenterol. 2000;6:377-380. [PubMed] [DOI] |
| 12. | Song YH, Lin JS, Liu NZ, Kong XJ, Xie N, Wang NX, Jin YX, Liang KH. Anti-HBV hairpin ribozyme-mediated cleavage of target RNA in vitro. World J Gastroenterol. 2002;8:91-94. [PubMed] [DOI] |
| 13. | Lee PA, Blatt LM, Blanchard KS, Bouhana KS, Pavco PA, Bellon L, Sandberg JA. Pharmacokinetics and tissue distribution of a ribozyme directed against hepatitis C virus RNA following subcutaneous or intravenous administration in mice. Hepatology. 2000;32:640-646. |
| 14. | Jia ZS, Zhou YX, Lian JQ, Feng ZH, Li GY. Inhihation of the expression of hepatitis C virus by hammerhead ribozyme in HHCC. Cell line. Zhonghua Yixue Zazhi. 1999;79:631-633. |
| 15. | Jia ZS, Zhou YX, Feng ZH, Lian JQ, Li GY, Jiao CS, Li GY. Intracellar inhibation of viral gene expression by ribozyme against 5'noncoding region of hepatitis C virus. Zhonghua Chuanranbing Zazhi. 2000;18:10-12. |
| 16. | Yamamoto M, Hayashi N, Takehara T, Ueda K, Mita E, Tatsumi T, Sasaki Y, Kasahara A, Hori M. Intracellular single-chain antibody against hepatitis B virus core protein inhibits the replication of hepatitis B virus in cultured cells. Hepatology. 1999;30:300-307. [PubMed] [DOI] |
| 17. | zu Putlitz J, Skerra A, Wands JR. Intracellular expression of a cloned antibody fragment interferes with hepatitis B virus surface antigen secretion. Biochem Biophys Res Commun. 1999;255:785-791. [PubMed] [DOI] |
| 18. | Chen L, Li G, Tang L, Wang J, Ge XR. The inhibition of lung cancer cell growth by intracellular immunization with LC-1 ScFv. Cell Res. 2002;12:47-54. [PubMed] [DOI] |
| 19. | Oka Y, Akbar SM, Horiike N, Joko K, Onji M. Mechanism and therapeutic potential of DNA-based immunization against the envelope proteins of hepatitis B virus in normal and transgenic mice. Immunology. 2001;103:90-97. [DOI] |
| 20. | Li JG, Zhou YX, Lian JQ, Jia ZS, Feng ZH. Inhibation of E antigen of hepatitis B virus by ribozyme in HHCC cell lines. Shijie Huaren Xiaohua Zazhi. 1999;7:28-30. [DOI] |
| 21. | Pertl U, Luster AD, Varki NM, Homann D, Gaedicke G, Reisfeld RA, Lode HN. IFN-gamma-inducible protein-10 is essential for the generation of a protective tumor-specific CD8 T cell response induced by single-chain IL-12 gene therapy. J Immunol. 2001;166:6944-6951. [PubMed] [DOI] |
| 22. | Fraisier C, Irvine A, Wrighton C, Craig R, Dzierzak E. High level inhibition of HIV replication with combination RNA decoys expressed from an HIV-Tat inducible vector. Gene Ther. 1998;5:1665-1676. [PubMed] [DOI] |
| 23. | Teixeira R, Papatheodoridis GV, Burroughs AK. Management of recurrent hepatitis C after liver transplantation. J Viral Hepat. 2001;8:159-168. [PubMed] [DOI] |
| 24. | Macejak DG, Jensen KL, Pavco PA, Phipps KM, Heinz BA, Colacino JM, Blatt LM. Enhanced antiviral effect in cell culture of type 1 interferon and ribozymes targeting HCV RNA. J Viral Hepat. 2001;8:400-405. [PubMed] [DOI] |
| 25. | Lee PA, Blatt LM, Blanchard KS, Bouhana KS, Pavco PA, Bellon L, Sandberg JA. Pharmacokinetics and tissue distribution of a ribozyme directed against hepatitis C virus RNA following subcutaneous or intravenous administration in mice. Hepatology. 2000;32:640-646. [PubMed] [DOI] |