修回日期: 2003-04-10
接受日期: 2003-04-16
在线出版日期: 2003-11-15
探讨通过反义Smad4基因转移阻断TGF-β1的信号传导后对贮脂细胞激活和细胞外基质产生的影响.
将Smad4的基因序列反向插入腺病毒表达质粒pAdv 5SR(+), 构建反义Smad4的表达质粒pAdAT Smad4. 该表达载体再与重组质粒pJM17同源重组, 共转染入293细胞, 通过PCR法筛选、鉴定, 得到含反义Smad4基因的复制缺陷型重组腺病毒AdAT Smad4. 将AdATSmad4扩增纯化, 再转入贮脂细胞株CFSC内, 应用RT-PCR检测反义基因的表达, 用原位杂交和免疫组织化学等方法检测Smad4和细胞外基质的产生.
转基因的CFSC细胞内有反义Smad4表达, 且其合成分泌Smad4和细胞外基质降低.
反义Smad4 RNA可以抑制贮脂细胞的激活和内源性Smad4和细胞外基质的产生, 为抗纤维化基因治疗提供理论依据.
引文著录: 徐新保, 冷希圣, 何振平, 梁志清. 贮脂细胞Smad4反义基因转移及对细胞外基质合成的抑制作用. 世界华人消化杂志 2003; 11(11): 1690-1693
Revised: April 10, 2003
Accepted: April 16, 2003
Published online: November 15, 2003
To investigate possible role of antisense Smad4 RNA in the regulation of Smad4 and ECM production in Ito cells after blockade of TGF-β1 signal transmission by antisense Smad4.
A rat Smad4 cDNA (2.5 kb) was inserted in reverse orientation into the adenoviral shuttle vector pAdv5SR (+), and so pAdvATSmad4 was obtained. PAdvATSmad4 was transfected, together with pJM17, into 293 cells by a liposome-mediated technique. We acquired the recombinant virus (AdvATSmad4) containing the anti-Smad4 gene by PCR detecting method. AdvATSmad4 was amplified and purified and then introduced into the rat Ito cell line CFSC.
The presence of antisense Smad4 RNA was detected by RT-PCR. The expression of Smad4 and the production of extracellular matrix were markedly decreased in the antisense Smad4 transfected cultured cells by in situ hybridization and immunohistochemistry.
Antisense RNA of Smad4 can be used successfully to inhibit Ito cell activation, endogenous Smad4 mRNA and extracellular matrix production, and may provide a basis for the development of anti-fibrosis gene therapy.
- Citation: Xu XB, Leng XS, He ZP, Liang ZQ. Smad4 antisense gene transfer into ito cells and suppressed extracellular matrix production. Shijie Huaren Xiaohua Zazhi 2003; 11(11): 1690-1693
- URL: https://www.wjgnet.com/1009-3079/full/v11/i11/1690.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i11.1690
转化生长因子-β1 (TGF-β1)在肝纤维化的发生、发展中起重要作用[1], 他既能激活Ito细胞合成细胞外基质(ECM), 又能抑制肝细胞的再生[2,3]. 同时Ito细胞又能表达TGF-β1, 通过自分泌和旁分泌作用, 使TGF-β1呈正反馈性增加和ECM大量合成, 加速肝纤维化的进程[4,5], 但TGF-β1的信号传导需要Smad蛋白分子参与(Smad2、Smad3和Smad4), 其中Smad4是最关键和必不可少的蛋白分子[6,7]. 为了阻断TGF-β1的信号传导我们构建了反义Smad4基因的表达载体, 并导入Ito细胞系CFSC内, 观察了其抑制Smad4表达及细胞外基质合成的效应, 现将结果报告如下.
克隆于重组腺病毒载体的多克隆位点的含有鼠反义Smad4 cDNA质粒载体pAdvATSmad4由本室构建. 腺病毒包装细胞293细胞为第三军医大学西南医院烧伤研究所王明海博士惠赠, 肝纤维化大鼠的贮脂细胞系(SFSC)由美国Albert Einstein College of Medicine的Marcos Rojkind惠赠[8]. 脂质体转染试剂盒、地高辛标记DNA及检测试剂盒购自Boehringer Madnnhem公司. Western blot化学发光试剂盒、Smad4抗体、I型胶原和IV型胶原抗体均购自Santa Cruz生物技术公司.
用脂质体介导反义Smad4重组腺病毒载体(pAdvATSmad4)和相应的空载体pAdv5SR(+), 分别与pJM17同源重组, 共转染入293细胞. PCR法筛选阳性克隆, 获得重组腺病毒AdvATSmad4, 内含反义Smad4 cDNA, 同时获得空病毒Adv0. 用293细胞扩增AdvATSmad4和Adv0, 采用反复冻融法纯化腺病毒. 用293细胞的细胞病变效率检测病毒滴度. 贮脂细胞分设正常组(CFSC)、空病毒转染组(CFSC/Adv0)、反义Smad4腺病毒转染组(CFSC/AdvATSmad4), 正常组以培养液处理, 后两组分别感染50 MOI 的Adv0和AdvATSmad4, 病毒感染24 h后换液, 继续培养24 h后, 收集细胞检测指标. 细胞RNA的提取按试剂盒说明书进行, PCR引物设计, Smad4 cDNA上游引物P1; 5'-TGG ACA TTA CTG GCC GGT TCA CAA-3'; 下游引物P2; 5'-CTC AAT CCA GCA CGG GGT TTC TTT-3'. 可扩出685bp的Smad4 cDNA条带. (3) RT-PCR检测反义Smad4表达; 反转录PCR (RT-PCR)按试剂盒说明书操作, 此时仅加P2引物, 其产物再行 PCR扩增(同时加P1、P2), 条件如下(PE公司) 9600型PCR仪; 94 °C 5 min→加Taq酶→94 °C 1 min, 56 °C 1 min, 72 °C 2 min, 共30个循环→72 °C延伸10 min→4 °C保存. 采用地高辛探记的Smad4 cDNA (1.2 kb)探针进行原位杂交, 检测CFSC, CFSC/Adv0, CFSC/AdvATSmad4等各组细胞的Smad4基因表达. 采用Western Blot (化学发光法)检测上述三组细胞的Smad4蛋白. 参照说明书进行. PT-PCR检测TGF-β1 的mRNA表达. 引物设计, TGF-β1 cDNA上游引物P1; 5'-GGG ACT ATC CAC CTG CAA GA-3'; F游引物P2; 5'-GAT CTT GAT CTT CAT GGT GCT AG-3'; 能扩出939 bp和648 bp的cDNA条带. 看家基因β-actin上游引物P3; 5'-TTG TAA CCA CCT GGG ACG ATA TGG-3'; 下游引物P4; 5'-GAT CTT GAT CTT CAT GGT GCT AG-3'; 能扩出768 bp的cDNA条带. 免疫组化检测I、IV型胶原的变化.
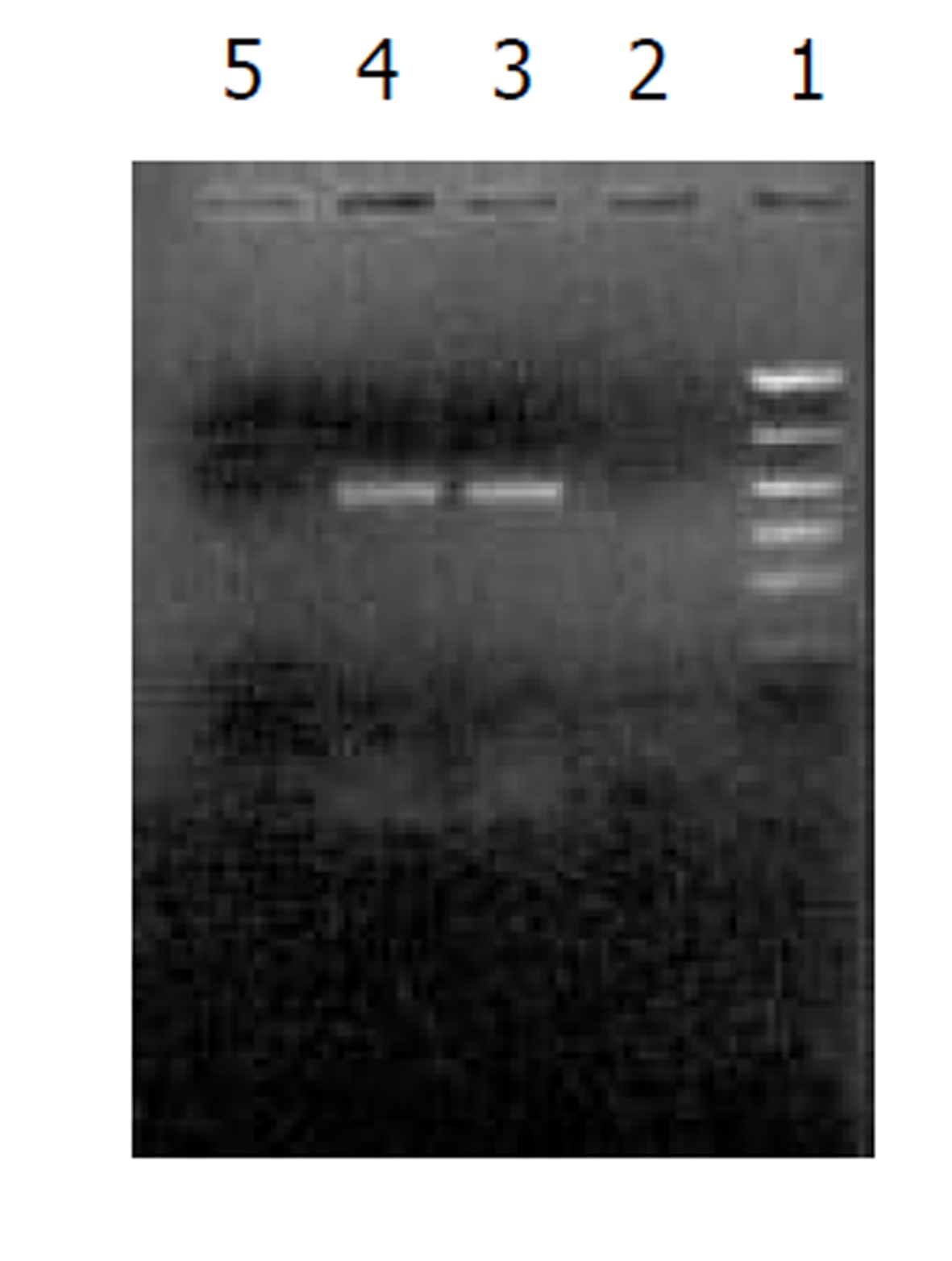
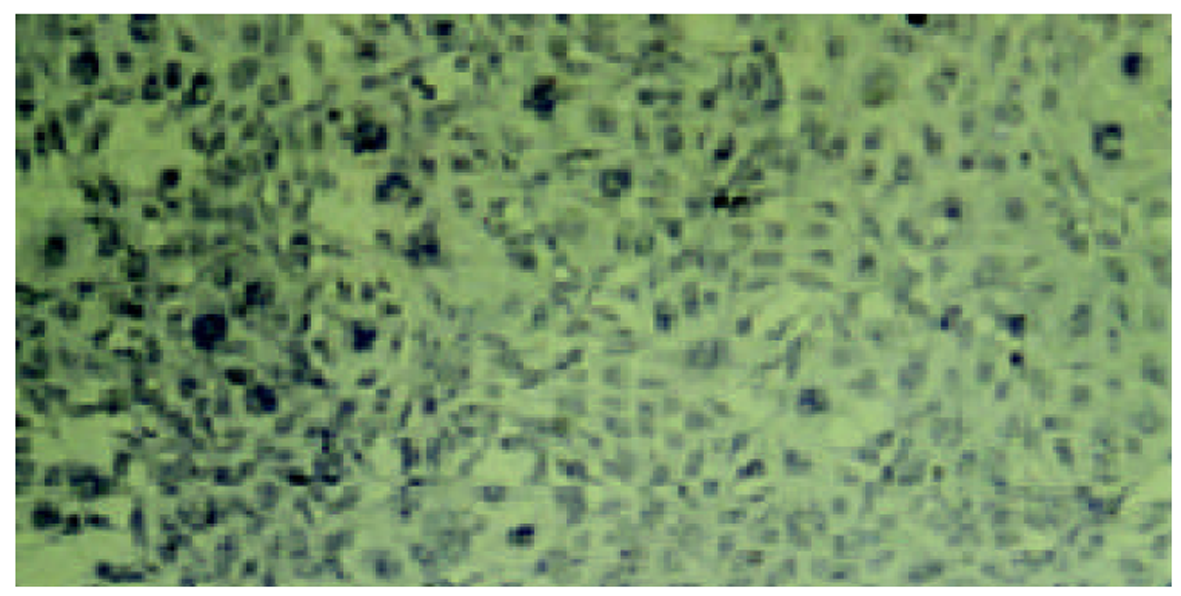
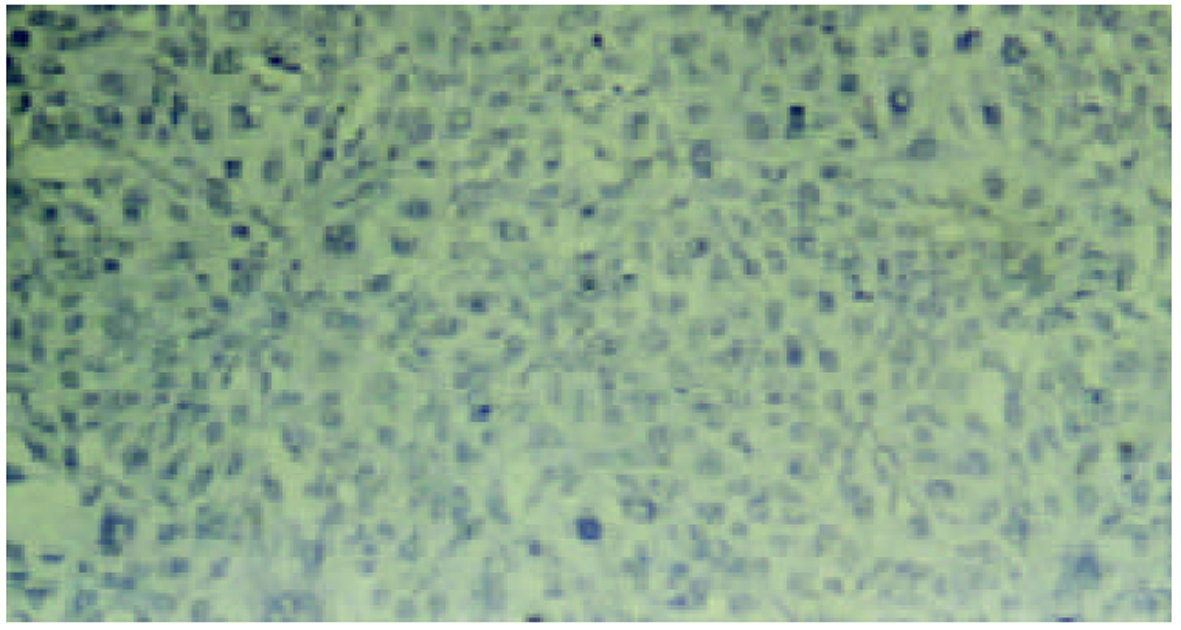
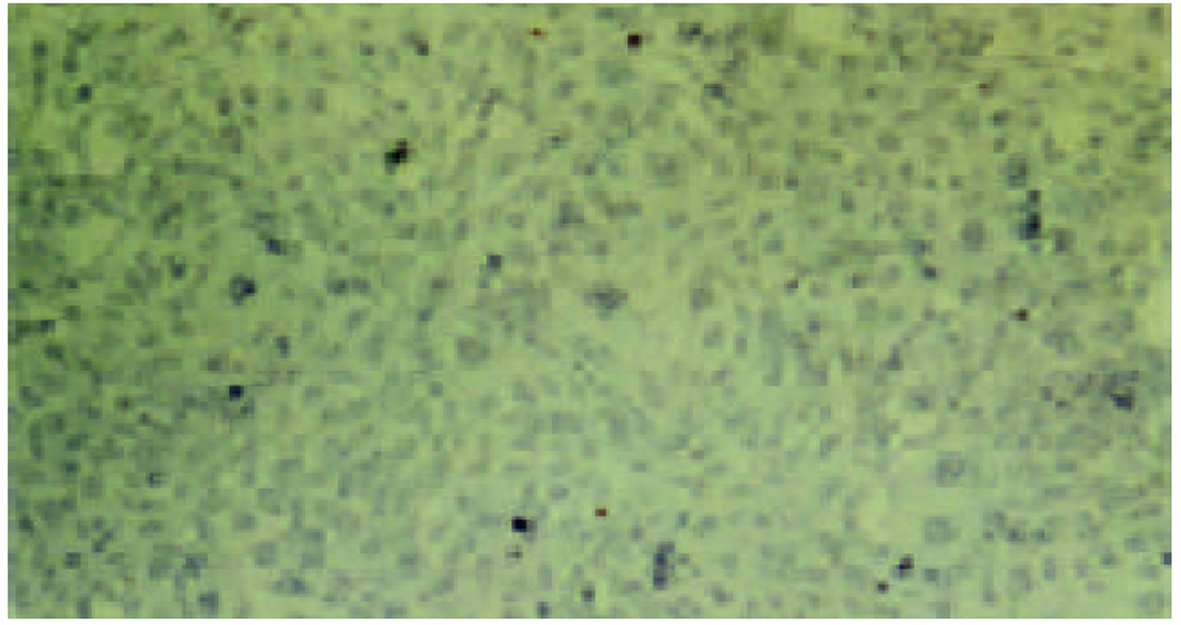
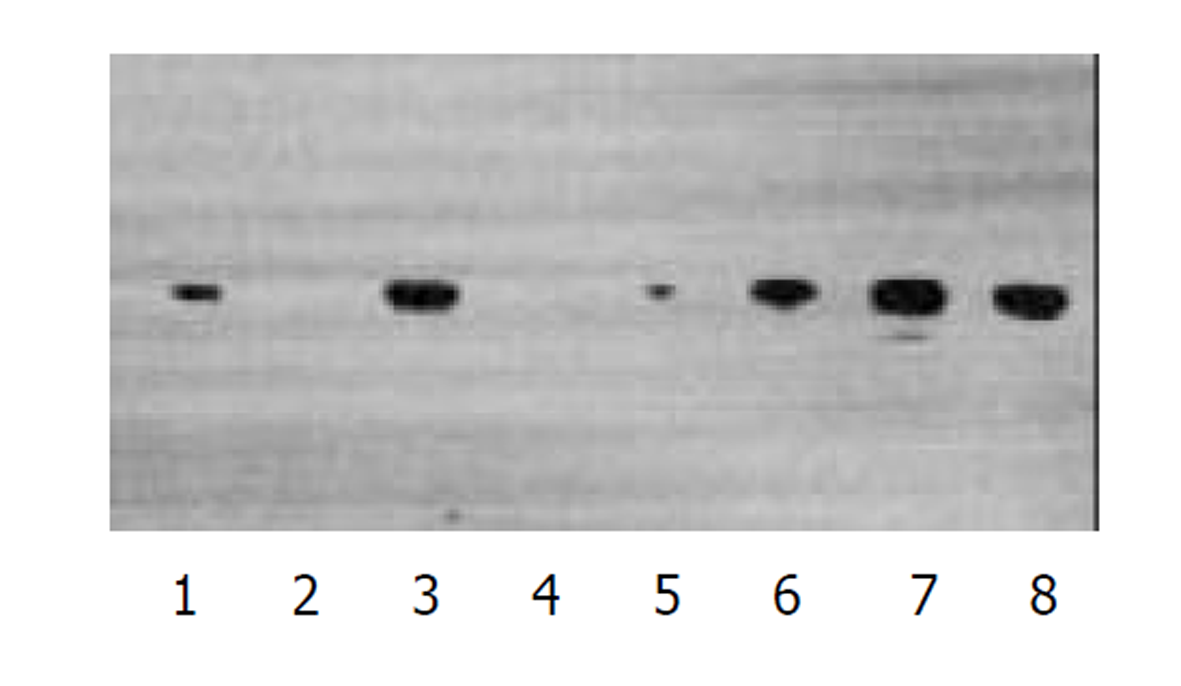
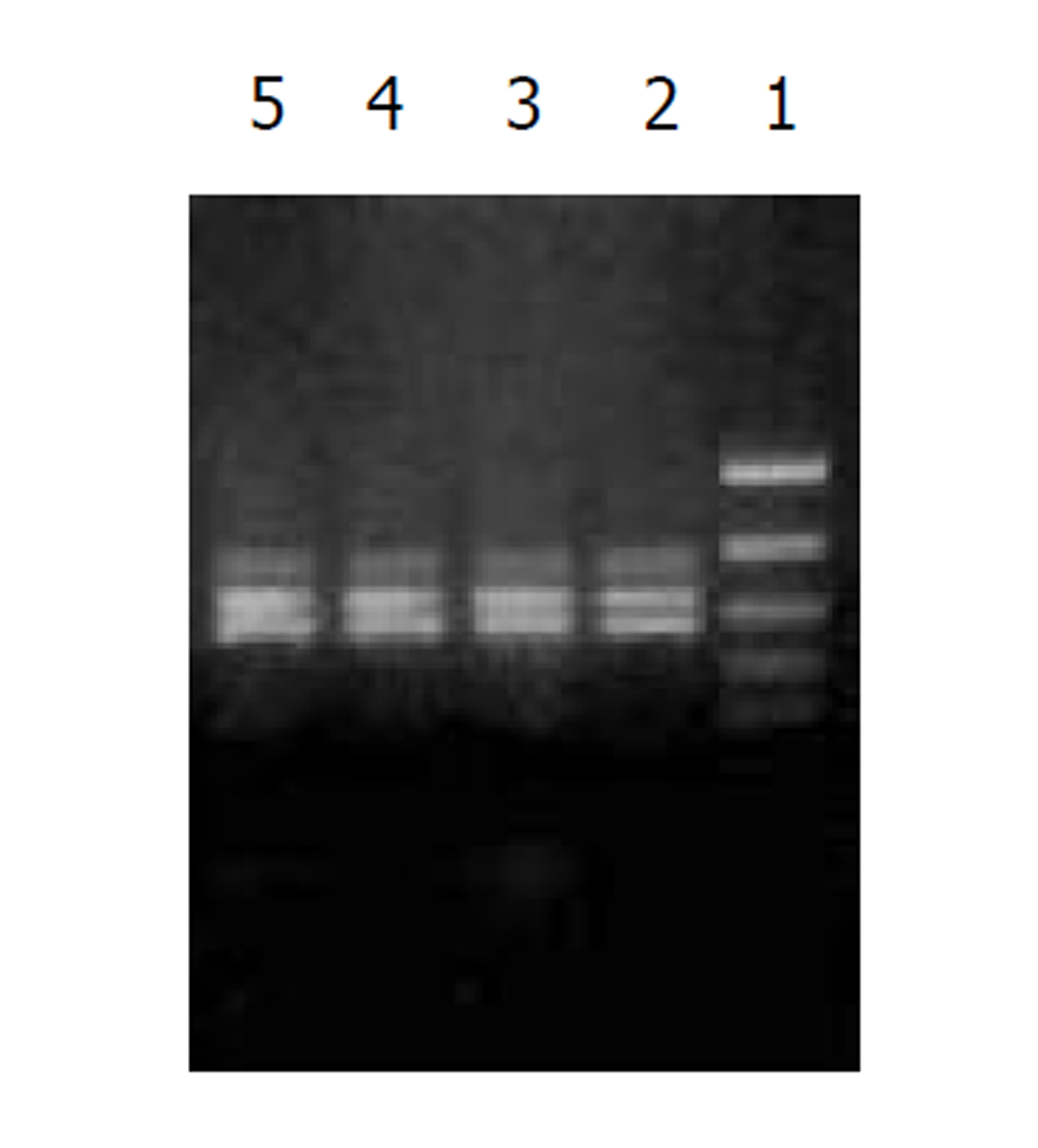
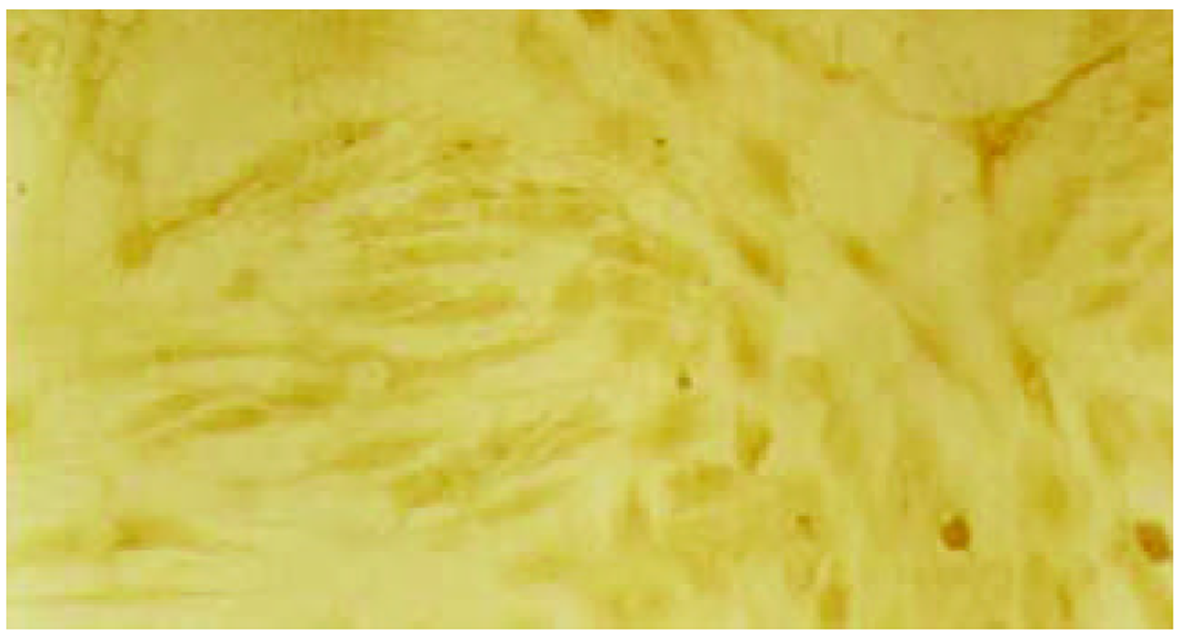
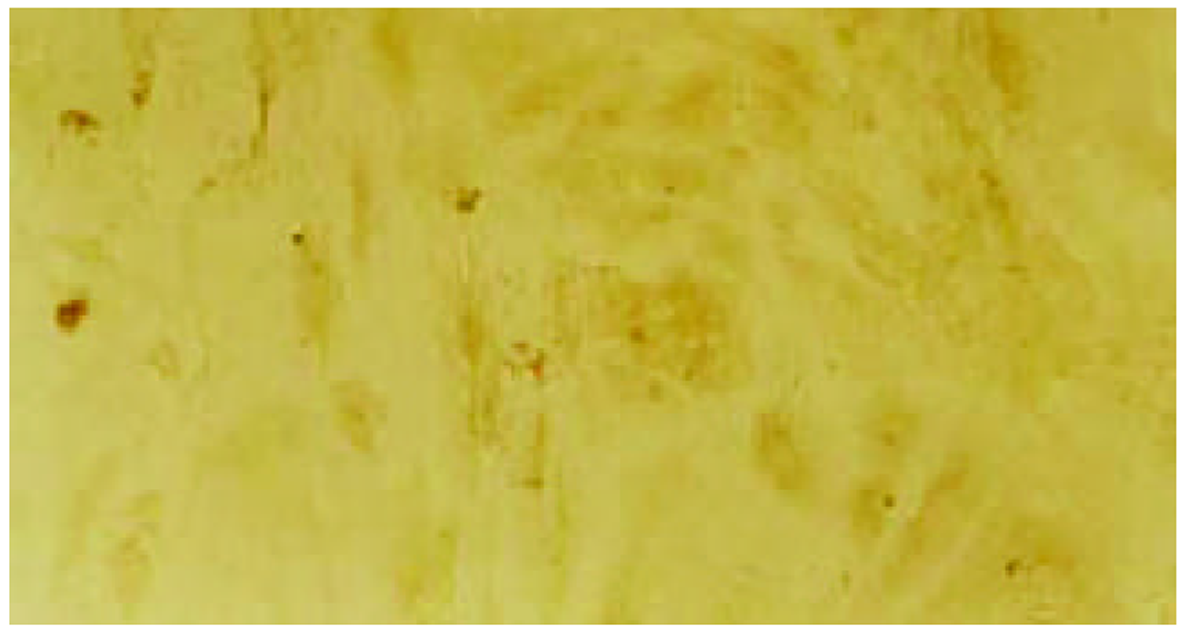
经测定AdvATSmad4和 Adv0的滴度均为5.0×1010 nfu/L. CFSC/AdvATSmad4细胞RNA的RT-PCR可扩出685 bp的反义Smad4条带, 而CFSC、CFSC/Adv0则未扩出该片段(图1). 原位杂交示CFSC/AdvATSmad4的Smad4 mRNA表达明显弱于CFSC, 而CFSC/Adv0与 CFSC相比无明显差别(图2, 图3, 图4). Western blot显示了类似的结果(图5). RT-PCR示CFSC、CFSC/AdvATSmad4均能扩出939 bp和648 bp的TGF-β1条带, 且强弱变化不明显, β-actin扩出768 bp条带(图6). 免疫组化显示CFSC/AdvATSmad4的I型胶原蛋白的阳性程度明显低于CFSC, (图7, 图8). IV型胶原蛋白的免疫组化结果类似于I型胶原.
肝纤维化为细胞外基质(ECM)过度沉积所致, 其发生与肝脏中ECM的合成增多及降解减少有关[9]. 研究表明, 肝脏中的ECM主要由肝间质细胞之一的肝星状细胞(HSC)产生[10]. 肝星状细胞(HSC)的激活与转化是肝纤维化形成的中心环节, 在肝纤维化的发生、发展中发挥了重要作用[10,11]. 一些细胞因子、生长因子通过调控肝星状细胞ECM的合成与分泌在肝纤维化形成中起作用, 其中TGF-β1是最重要的促进肝纤维化的生长因子[9,12].
贮脂细胞系CFSC (Fat-storing cell lines from CCl4-cirrhotic liver) 1991年建系[8]. 该细胞株是从肝硬化大鼠(CCl4腹腔注射5 wk)的肝脏中分离培养而得到的连续细胞系. 在mRNA水平和蛋白水平, 该细胞系表达I、III、IV型胶原, 纤维连接蛋白, TGF-β, 白介素-6, 白介素-8和TNF-α[13-15] 以及NF-kappaB[16]. 经我们检测, 该细胞也表达Smad4的mRNA及蛋白, 因而非常适宜于本实验. 腺病毒具有产生的滴度高、感染效率高(既能感染静止期细胞, 又能感染分裂期细胞)、不整合入染色体基因组、不易引起插入突变等优点[17], 已经被广泛应用于肝纤维化的实验治疗研究并且获得了比较理想的效果[18-22], 因此, 本研究采用了腺病毒作为载体.
在哺乳动物组织中存在三种形式的TGF-β, 分别是TGF-β1、TGF-β2、TGF-β3. 他们位于不同的染色体上, 由不同的细胞分泌和表达. 其中, TGF-β1所占比例最高(大于90%), 活性最强[23]. TGF-β1 有三种受体, 被分别被命名为I型、II型和III型受体, 他们存在于所有的细胞中, I型、II型受体是跨膜丝氨酸/ 苏氨酸激酶[24]; III型受体无信号转导结构但可递呈TGF-β1给其他受体, I型受体介导TGF-β1 的细胞外基质的合成和降解作用, II型受体介导TGF-β1 的细胞生长和分化作用[5].
TGF-β1的激活分四个步骤; (1) TGF-β1首先与辅助性的TGF-βIII型受体 (TβR-III) 结合; (2) 随后, TGF-β1与TGF-βII型受体(TβR-II)结合, 并与TGF-βI型受体(TβR-I)结合形成异源复合物, 包括两个TβR-IIs和两个TβR-Is; (3) 活化的TβR-II激酶磷酸化TβR-I; (4) TβR-I通过特定的Smads分子传导TGF-β1的信号[25]. Smads是近几年发现的一类TGF-β的下游细胞内信号传导蛋白, 包括8个成员, 即Smad1-8. 他们可被合为三类; (1) 受体激活Smads (receptor-activated smads, R-smads). (2) 共同伴侣Smads (common-partner smads, co-smads). (3) 抑制性Smads (inhibitory smads, anti-smads). R-Smads包括Smad1、2、3、5和8. 其中, Smad1、5、8传导BMP的信号, 而Smad2、3传导TGF-β[10,11,26]和激活素(activin)的信号[27]. Co-Smads是Smad4, 他是R-Smads传导信号的伴侣[28]. R-Smads传导信号必须先与Smad4结合形成异源复合物, 才能进到核中, 调节转录活动. Anti-smads包括Smad6和Smad7, 他们抑制R-Smads传导信号. 由此可见, 在TGF-β的信号传导中, Smad4起着关键性的作用[28,29].
因为TGF-β1在肝纤维化中起重要作用[1-5,29], 因此, 抑制TGF-β1的活性, 可以阻止肝纤维化的进程[30-32]. 也有报道通过抑制TGF-βI型[32]和II型受体活性的方法来消弱TGF-β的信号传导通路, 从而显著减轻了二乙基亚硝胺所诱导的大鼠肝纤维化程度.
因此, 通过反义Smad4封闭贮脂细胞内源性Smad4的产生, 消弱TGF-β1的信号传导, 能够达到抑制TGF-β1活性的目的. 我们采用反义技术, 将Smad4基因反向构建于腺病毒载体, 用脂质体介导, 与PJM17共转染包装细胞293细胞, 获得重组腺病毒AdvATSmad4, 将其感染贮脂细胞CFSC, 使其在基因转录、翻译水平阻止CFSC的Smad4表达. 通过RT-PCR发现反义Smad4已在CFSC/AdvATSmad4中表达, 而转染空病毒的CFSC/Adv0和未转基因CFSC中无反义Smad4表达. 原位杂交发现; CFSC/AdvATSmad4的Smad4的RNA表达明显低于CFSC/Adv0和CFSC, 表明反义Smad4RNA能使贮脂细胞合成分泌Smad4减少. RT-PCR显示反义Smad4对CFSC的TGF-β1表达无明显直接影响. 免疫组化表明反义Smad4能减少CFSC的I、IV型胶原合成. 因此, 我们认为反义Smad4RNA不仅可以阻止贮脂细胞内源性Smad4的合成, 还能阻止或减少贮脂细胞细胞外基质的合成, 以减缓或阻止肝纤维化的进程, 这种作用主要是通过阻断或消弱TGF-β1的信号传导而实现的.
| 2. | Albanis E, Safadi R, Friedman SL. Treatment of hepatic fibrosis: almost there. Curr Gastroenterol Rep. 2003;5:48-56. [DOI] |
| 3. | Lietard J, Loreal O, Theret N, Campion JP, L'Helgoualc'h A, Turlin B, Ramee MP, Yamada Y, Clement B. Laminin isoforms in non-tumoral and tumoral human livers. Expression of alphal, alpha2, betal, beta2 and gamma1 chain mRNA and an alpha chain homologous to the alpha2 chain. J Hepatol. 1998;28:691-699. [PubMed] |
| 4. | Bachem MG, Meyer D, Melchior R, Sell KM, Gressner AM. Activation of rat perisinusoidal lipocytes by transforming growth factor derived from myofibroblastlike cells: A potential mechanism of self perpetuation in liver fibrogenesis. J Clin Invest. 1992;89:19-27. [DOI] |
| 6. | Heldin CH, Miyazonok K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465-471. [DOI] |
| 7. | Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359-4369. [PubMed] |
| 8. | Greenwel P, Schwartz M, Rosas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991;65:644-653. [PubMed] |
| 9. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:793-807. [DOI] |
| 10. | Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K, Xu L, Eng F, Afdhal N, Kalluri R. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147-159. [DOI] |
| 11. | Dooley S, Delvoux B, Streckert M, Bonzel L, Stopa M, ten Dijke P, Gressner AM. Transforming growth factor beta signal transduction in hepatic stellate cells via Smad2/3 phosphorylation, a pathway that is abrogated during in vitro progression to myofibroblasts. TGFbeta signal transduction during transdifferentiation of hepatic stellate cells. FEBS Lett. 2001;502:4-10. [DOI] |
| 12. | Bissell DM. Chronic liver injury, TGF-beta, and cancer. Exp Mol Med. 2001;33:179-190. [DOI] |
| 13. | Hernandez E, Correa A, Bucio L, Souza V, Kershenobich D, Gutierrez-Ruiz MC. Pentoxifylline diminished acetaldehyde-induced collagen production in hepatic stellate cells by decreasing interleukin-6 expression. Pharmacol Res. 2002;46:435-443. [DOI] |
| 14. | Freeman TL, Kharbanda KK, Tuma DJ, Mailliard ME. Inhibition of hepatic stellate cell collagen synthesis by N-(methylamino)isobutyric acid. Biochem Pharmacol. 2002;63:697-706. [DOI] |
| 15. | Gutierrez-Ruiz MC, Bucio L, Correa A, Souza V, Hernandez E, Gomez-Quiroz LE, Kershenobich D. Metadoxine prevents damage produced by ethanol and acetaldehyde in hepatocyte and hepatic stellate cells in culture. Pharmacol Res. 2001;44:431-436. [DOI] |
| 16. | Vasiliou V, Lee J, Pappa A, Petersen DR. Involvement of p65 in the regulation of NF-kappaB in rat hepatic stellate cells during cirrhosis. Biochem Biophys Res Commun. 2000;273:546-550. [DOI] |
| 17. | Zhang L, Graziano K, Pham T, Logsdon CD, Simeone DM. Adenovirus-mediated gene transfer of dominant-negative Smad4 blocks TGF-beta signaling in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1247-1253. [DOI] |
| 18. | Song E, Chen J, Wang K, Zhang H, Su F, Wang M, Heemann U. Intrasplenic transplantation of syngenic hepatocytes modified by IFN-gamma gene ameliorates hepatic fibrosis in rats. Transpl Int. 2002;15:472-478. [DOI] |
| 19. | Iimuro Y, Nishio T, Morimoto T, Nitta T, Stefanovic B, Choi SK, Brenner DA, Yamaoka Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445-458. [DOI] |
| 20. | Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125:178-191. [DOI] |
| 21. | Suzuki K, Aoki K, Ohnami S, Yoshida K, Kazui T, Kato N, Inoue K, Kohara M, Yoshida T. Adenovirus-mediated gene transfer of interferon alpha improves dimethylnitrosamine-induced liver cirrhosis in rat model. Gene Ther. 2003;10:765-773. [DOI] |
| 22. | Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287:1253-1258. [DOI] |
| 23. | Bissell DM, Roulot D, George J. Transforming growth factor beta and the liver. Hepatology. 2001;34:859-867. [DOI] |
| 24. | Ebner R, Chen RH, Lawler S, Zioncheck T, Derynck R. Determination of type I receptor specificity by the type II receptors for TGF-beta or activin. Science. 1993;262:900-902. [DOI] |
| 25. | Persson U, Izumi H, Souchelnytskyi S, Itoh S, Grimsby S, Engstrom U, Heldin CH, Funa K, ten Dijke P. The L45 loop in type I receptors for TGF-β family members of a critical determinant in specifying smad isoform activation. FEBS Lett. 1998;434:83-87. [DOI] |
| 26. | Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, Brenner DA. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology. 2001;34:89-100. [DOI] |
| 27. | Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten Dijke P. TGF-β receptor-mediated signalling through smad 2, Smed3 and Smad4. EMBO J. 1997;16:5353-5362. [DOI] |
| 28. | Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp Cell Res. 2003;282:90-100. [DOI] |
| 30. | Arias M, Lahme B, Van de Leur E, Gressner AM, Weiskirchen R. Adenoviral delivery of an antisense RNA complementary to the 3 coding sequence of transforming growth factor-beta1 inhibits fibrogenic activities of hepatic stellate cells. Cell Growth Differ. 2002;13:265-273. [PubMed] |
| 32. | Liu X, Zhang Z, Yang L, Chen D, Wang Y. Inhibition of the activation and collagen production of cultured rat hepatic stellate cells by antisense oligonucleotides against transforming growth factor-beta 1 is enhanced by cationic liposomedelivery. Huaxi Yike Daxue Xuebao. 2000;31:133-135. |