修回日期: 2003-08-20
接受日期: 2003-09-01
在线出版日期: 2003-10-15
建立清除乙型肝炎病毒(HBV)自杀基因平衡制约载体系统, 靶向清除HBV感染的肝细胞, 抑制慢性乙型肝炎和肝硬化患者免疫再活动.
逆转录PCR(RT-PCR)扩增丙型肝炎病毒(HCV)基因组中核糖体插入位点(IRES), 以pcDNA3载体为基础, 构建双顺反子表达载体. 同时在IRES位点的上游克隆HBV表面基因的部分反义序列和HCV 全长核心基因, 在IRES位点下游克隆胸腺苷激酶基因, PCR、酶切和测序鉴定. 构建的清除乙型肝炎病毒(HBV)自杀基因平衡制约载体系统分别转染培养基含有更昔洛韦的HepG2细胞和2.2.15细胞.
构建的清除乙型肝炎病毒(HBV)自杀基因平衡制约载体系统可以选择性促使2.215细胞凋亡(实验组细胞凋亡率为15%, 而对照组仅为6%); 抑制2.2.15细胞在培养基中分泌乙型肝炎病毒表面抗原(HBsAg), 统计学分析表明, HBsAg在培养基中检测滴度, 在两组之间有显著性差异(P<0.05).
构建以HBV 表面基因为靶基因, 自杀基因为效应基因的自杀基因制约平衡系统, 可能为下一步慢性乙型肝炎的基因治疗提供理想的基因治疗载体.
引文著录: 阚全程, 余祖江, 雷延昌, 杨东亮, 郝连杰. 慢性乙型肝炎病毒清除自杀基因平衡制约载体系统的构建. 世界华人消化杂志 2003; 11(10): 1515-1519
Revised: August 20, 2003
Accepted: September 1, 2003
Published online: October 15, 2003
To construct the vector that harbors self-restricted system for clearing hepatitis B virus, eliminating infected hepatic cells and inhibiting hepatitis B recurrence in gene therapy.
After amplifying hepatitis C virus (HCV) internal ribosome entry sites (IRES) by reverse-transcription PCR (RT-PCR), the products were cloned into pcDNA3. A biscistronic vector was obtained. A part of sequence in HBV anti-surface gene and part of sequence in HCV core gene were cloned into the vector before IRES site in turn and thymidine kinase (TK) was also cloned into it following the IRES site. After determination by PCR and sequencing, we acquired the vector containing HBV anti-S, HCV-C gene, HCV IRES and thymidine kinase gene, which was named the vector pcDNA3-SCITK. The vectors were separately transfected into HepG2 cells and 2.2.15 cells and all the media contained ganciclovir.
The novel vector was transfected into 2.2.15 and hepG2 cells, the expressed protein could destroy the former, but had no effect on HepG2 cells if all the media contained ganciclovir. Apoptosis cells in the former accounted for 15 per cent of all cells by fluorescence (FACS) detection. There was obvious difference between the two types of cells (the later was only 6 per cent).
The function of genes that pcDNA3-SCITK carried with self-restricted system could be ego-controlled, and it might be used as gene therapy vector for HBV clearance if taking HBV S gene as target gene and TK gene as objective gene.
- Citation: Kan QC, Yu ZJ, Lei YC, Yang DL, Hao LJ. Construction of the vector that harbors self-restricted system for hepatitis B virus clearance in gene therapy. Shijie Huaren Xiaohua Zazhi 2003; 11(10): 1515-1519
- URL: https://www.wjgnet.com/1009-3079/full/v11/i10/1515.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i10.1515
乙型肝炎病毒(hepatitis B virus, HBV)感染是威胁人类健康的严峻问题, 全球大约有5%的人受到感染, 我国一般人群的HBV表面抗原携带率高达10%[1-4]. 其中75-90%原发性肝癌与此有关, 因此研究控制HBV感染的有效方法, 一直是HBV的研究的难点和重点[5,6]. 当前人们主要根据上述慢性HBV感染3个自然过程及其相应的机体免疫应答特点[7-9], 进行乙型肝炎病毒清除的治疗研究. 迄今为止, 还没有一种有效的策略可以控制和消除免疫清除后的逃逸病毒和耐受期高水平复制的HBV[10,11]. 采用现代基因治疗方法, 有可能对这两个时期的低病毒复制和高水平复制HBV-DNA的细胞进行靶向性基因治疗, 影响着慢性乙型肝炎预后. 我们根据HCV复制特殊性, 结合乙型肝炎免疫应答的基本特点, 利用真核细胞表达载体, 构建基因功能平衡制约系统, 有可能为上述困境提供一种新的治疗方法.
pcDNA3, PCR产物提纯试剂盒, 德国QIAGEN; Expand PCR Kit, 美国Stratgene; Taq, Pfu酶, 加拿大Bio-Star; EcoR I, BamH I、HinD III, 洛阳华美生物工程公司. HBV pcDNA3-TK 和pcDNA3-S基因质粒系本室保存. 引物合成由大连宝生物公司合成. HCV IRES L: 5'- GCGC GGATCCGGGCGA CACTCCACCATAG -3' (nt17-36, 划线为BamH-I切点), HCV IRES R: 5'-GCGAATTCGTTTTTCTTT GAGGTTTAGGATTC -3' (nt347-371, 划线为EcoR I切点), HBVL: 5'-GCGCGTGCAAGCTTAT AAAACGCCGCAGACACATC-3'(划线为HinD III切点)HBVR: 5'-ATTCGTGCTCATCAGGATTCCT AGGACCCCTTC-3'(划线为HCV 核心基因), HCVL: 5'-TAGGAATCCTGATGAGCACGAA TCCTAAACCTC-3'(划线为HBV 部分反义序列), HCVR: 5'-GCGCGGATCCTTAAGCGGAAG CTGGG ATG-3'(划线为BamH I切点), TK R: 5'-ACTTCCGTGGCTTCTTGCTG-3'(nt150-nt170).
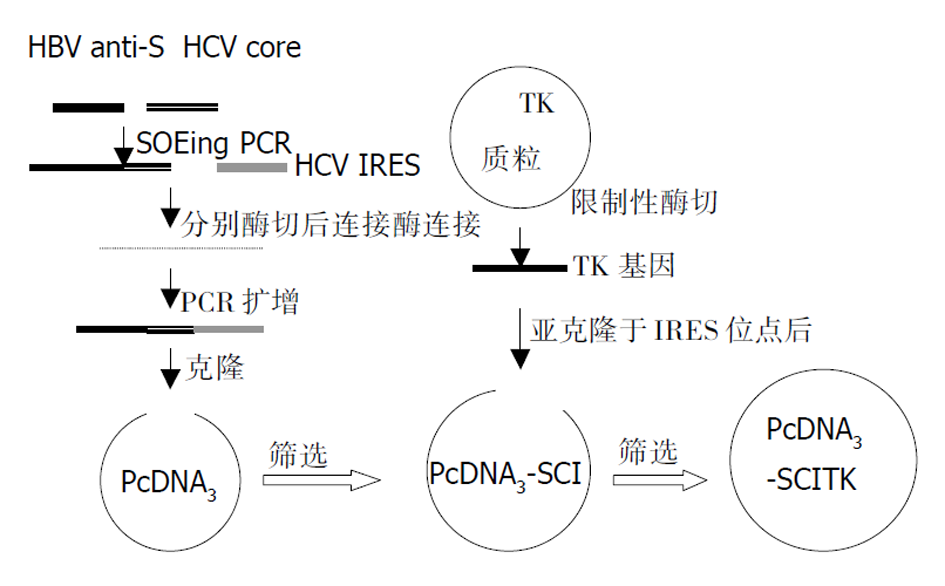
载体构建策略, 如图1, HCV IRES 位点和核心基因的获得. 临床上已被鉴定的1例国人慢性HCV感染患者的阳性血清50 μL, Trizol裂解(深圳晶美公司提供), 混匀后常规酚: 氯仿: 异戊醇抽提, 取上清2倍乙醇沉淀, 750 mL/L的乙醇洗涤, 沉淀烘干后加的DEPC水溶解20 μL, 获得HCV RNA. 取含有HCV RNA的溶液, 按照RT-PCR试剂盒(试剂提供为深圳晶美公司)要求, 分别加入随机引物, HCV IRES上下游引物, 含有逆转录酶的PCR扩增混合物. PCR仪为美国罗氏公司Real-time PCR仪. 反应程序为: 42 °C, 45 min; 94 °C, 5 min后进入主循环92 °C 30 s, 55 °C 30 s, 72 °C 30 s, 45个循环后收集PCR产物为目的HCV IRES目的基因产物. 同样的方法获得 HCV核心基因. 分别提纯后备用. HBV部分S基因和胸腺苷激酶基因获得. 根据HBV S基因序列, 寻找合适的反义序列, 在HBV S基因反义序列5'端处有一200 nt左右序列, 之中不含有起始密码子ATG, 设计引物(见上述). 按下列程序: 主循环92 °C 30 s, 55 °C 30 s, 72 °C 30 s PCR扩增, 产物为反义HBV S基因目的序列; 小量提取TK质粒, EcoR I酶切获得 TK基因. 分别提纯后备用. 反义 HBV S和HCV 核心基因连接: 按照Horton et al [12] (Gene 1989; 77: 61-68)方法进行SOEing PCR, 保守扩增. 在1.4.1.1中目的基因获得后, 反义 HBV S和HCV核心基因引物之间(HCV L和HBV R)存在互补序列(下划线所示): HCVL5-TAGGAATCCTGATGAGCACGAATCCTAAACCTC-3(斜体为HCV 核心基因序列)3-CCCCAGGATCCTTAGGACTACTCGTGCTTA-5: HBVR(斜体为反义HBV S基因部分). 所以, 将反义HBV S基因和HCV 核心基因扩增产物按一定的比例混合后, 以HBV L和HCV R为引物进行第二次PCR扩增, 反应按下列程序进行: 主循环92 °C 30 s, 50 °C 45 s, 72 °C 60 s PCR扩增30个循环后, 72 °C再延伸10 min, 获得目的基因产物, 提纯后备用. 反义HBV S-HCV 核心基因和HCV IRES位点三片段连接. BamH I分别常规酶切反义HBV S-HCV 核心基因和HCV IRES位点, 分别提纯后按一定的比例混合, 常规T4连接酶连接, 连接产物常规酚: 氯仿: 异戊醇抽提后获得上清. 取上清, 以HBV L和HCV IRES R为引物, 进行PCR扩增, 反应按下列程序进行: 主循环92 °C 30 s, 53 °C 45 s, 72 °C 60 s PCR扩增30个循环后, 72 °C再延伸10 min, 获得目的基因产物, 提纯后备用. 反义HBV S-HCV核心基因-HCV IRES三片段克隆. HinD III 和EcoR I双向酶切反义HBV S-HCV核心基因-HCV IRES序列和pcDNA3载体, 提纯, T4连接酶连接. 连接产物按常规方法进行转化感受态细胞后, 涂板, 37 °C培养, 挑取单菌落, 常规培养转化后的菌株, 以HBV L和HCV IRES R为引物进行PCR和HinD III和EcoR I双酶切鉴定, 获得的载体称为pcDNA3-SCI.
1.2.1 胸腺苷激酶基因亚克隆 EcoR I分别酶切pcDNA3-SCI和前面所得的TK基因, 提纯后T4连接酶连接, 连接产物按常规方法进行转化感受态细胞后, 涂板, 37 °C培养, 挑取单菌落, 常规培养转化后的菌株, 以HBV L和TK R为引物进行PCR扩增, 有目的产物出现即为TK基因正向插入pcDNA3-SCI载体后的目的质粒, 即清除乙型肝炎病毒自杀基因平衡制约载体系统(pcDNA3-SCITK). 获得的质粒送大连宝生物公司测序鉴定.
1.2.2 获得的载体细胞转染 常规培养细胞, 转染前1 d将HepG2和2.2.15细胞接种到8孔槽中, 加入100 mL/L胎牛血清( FCS)的DMEM培养基, 在37 °C和50 mL/L的CO2中培养至细胞的融合率为50-80%. 用QIAGENE小量提取质粒(pcDNA3-SCI和pcDNA3-SCITK), 按lipofectamine试剂盒(boehringer mannheim biochemicals Co. USA)说明书要求, 转染HepG2和2.2.15细胞, 0, 3, 6 d分别取细胞培养基上清, 6 d后, 细胞常规碘化丙啶色, 流式细胞仪检测.
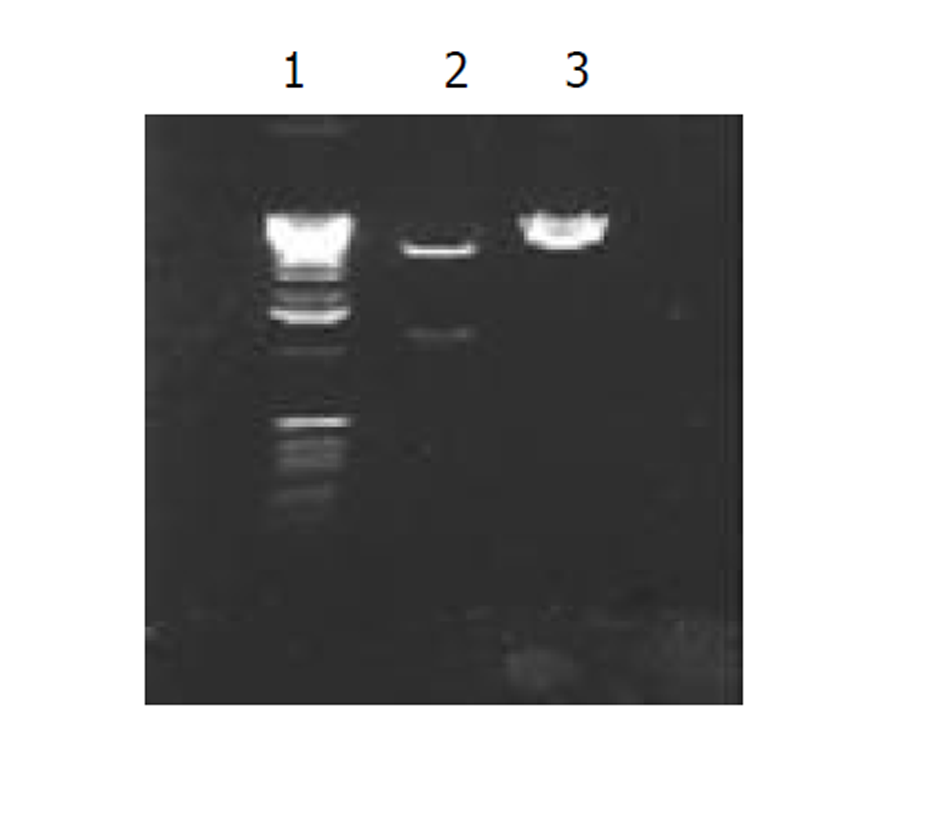
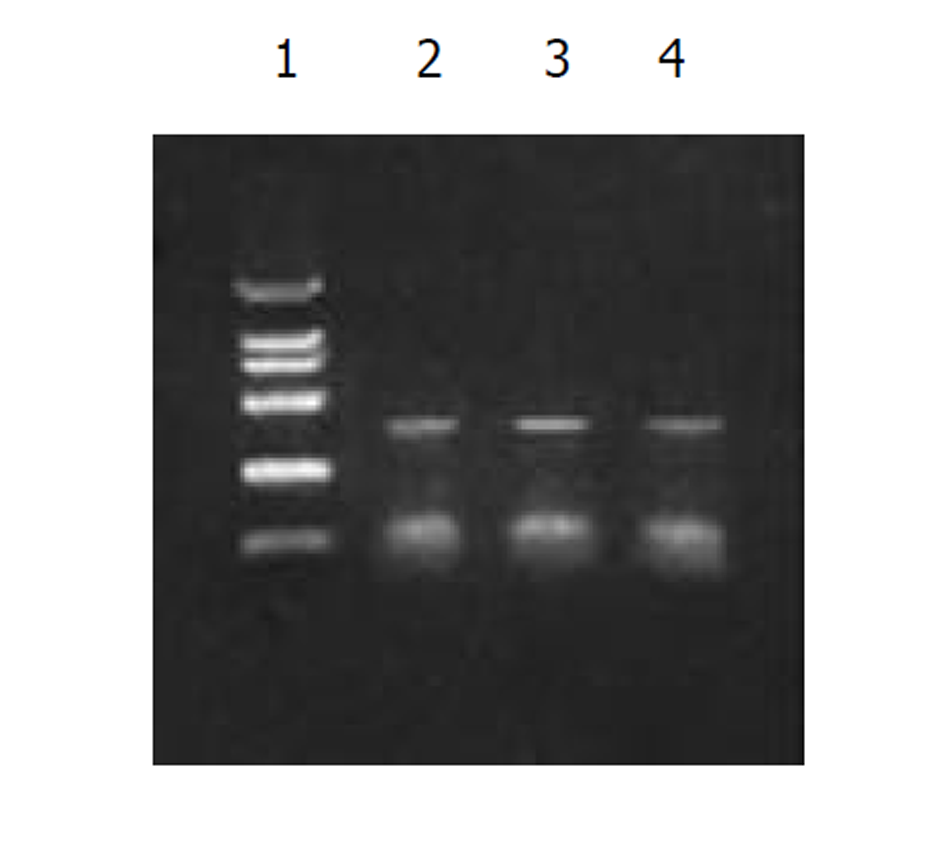
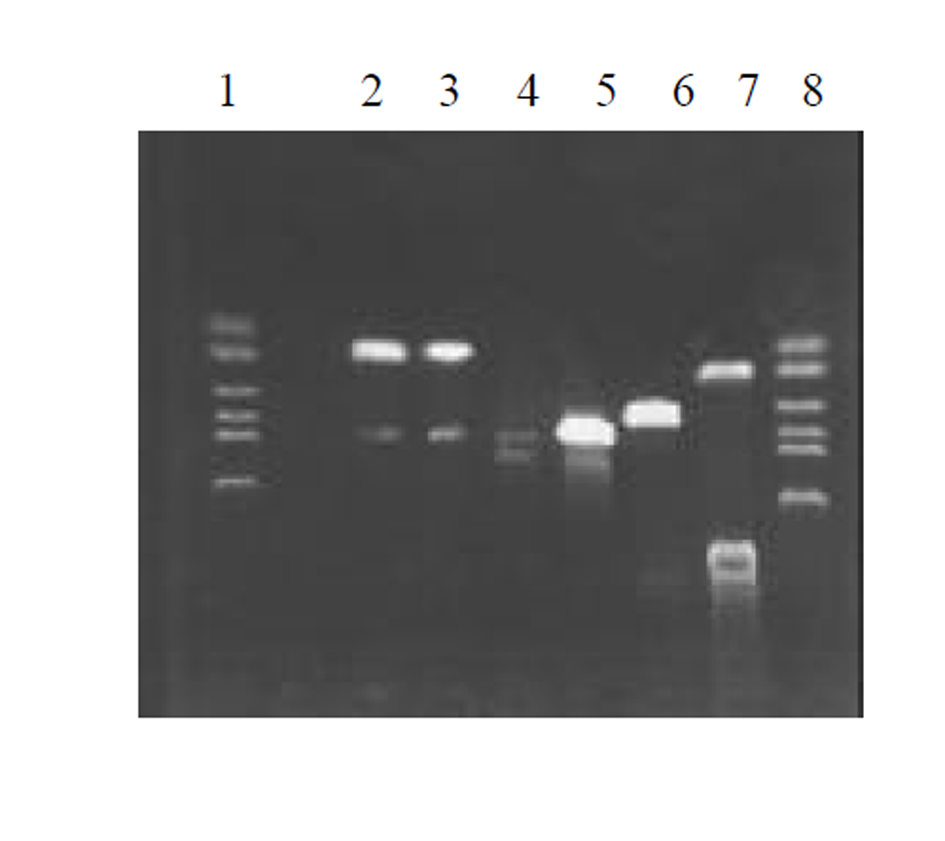
图2为 HCV IRES RT-PCR结果, 左侧为2 kb Mark, 右侧为PCR 产物, 大小300 bp左右; 图3为反义HBV S-HCV 核心基因-HCV IRES克隆后, HinD III和EcoR I双酶切鉴定结果, 左侧为 1 kb的Mark, 右侧为2泳道为双酶切结果, 前面为1 kb左右的SCI基因片段, 后面为线状pcDNA3载体, 3泳道为pcDNA3-SCI载体; 图4为SCITK平衡制约系统克隆和鉴定过程, 1和8泳道为2 kb的Mark, 2, 3泳道为SCITK载体系统经过HBV L和TK R为引物进行PCR扩增鉴定结果, 4泳道为反义HBV S-HCV 核心基因和HCV IRES位点连接后PCR扩增后提纯的电泳结果, 5泳道反义 HBV S和HCV 核心基因SOEing PCR连接的结果, 6泳道为HCV core RT-PCR结果, 7泳道为HBV 部分S基因PCR结果.
ELISA分析2.2.15细胞培养上清, 表达的HBsAg检测结果呈波浪性. 对照组(pcDNA3-SCI)1, 3, 6 d的A值分别为0.32±0.13, 0.61±0.23, 0.61±0.25, 而实验组(pcDNA3-SCITK)分别为0.31±0.11, 0.51±0.22, 0.41±0.16. 在检测结果6 d, 二者之间呈显著性差异 (P<0.05).
慢性乙型肝炎高水平的病毒复制、低水平的免疫应答和不完全免疫清除后逃逸的较少病毒复制是慢性乙型肝炎间断性发作的决定性因素. 目前常用的免疫调节剂如干扰素, IL-2等治疗效果不尽人意. 以疗效较为肯定干扰素-α为例, 其对e抗原的阴转率不到50%, 对HBsAg的阴转率几无影响, 而且远期疗效尚差, 副作用大, 价格昂贵[13-15]. 另外抗病毒药物拉咪呋定, 虽然有较强抑制病毒复制作用, 但停药后, 病毒又重新复制而复发[16-18]. 现有的抗HBV药物都不能清除HBV cccDNA, 停药后可以再度复制, 难以达到使其表达HBsAg消失, 因此远期治疗效果有限. 迄今为止, 还没有一种有效的策略可以控制和消除这些免疫清除后的逃逸病毒和耐受期高水平复制的HBV. 随着分子生物学研究进展, 通过基因治疗的方法来治疗慢性乙型肝炎已经成为目前研究的热点. 基因治疗的关键在于载体选择, 必须具有靶向性, 目的基因可调控性及有效表达等[19,20]. 因此对载体的合理修饰和改建是目前研究的热点和难点. 我们根据HCV复制特殊性, 蛋白质翻译的基本特点, 利用成熟的胸腺苷激酶基因(thymidine kinase, TK)为目的基因[21-25], 构建基因功能平衡制约系统, 使目的基因的可调控性和靶向性融为一体, 有可能为目前慢性乙型肝炎的基因治疗提供一个有效的载体.
在慢性病毒性肝炎的病原中, 除HBV外, 丙型肝炎病毒(hepatitis C virus, HCV)也是重要的病原之一. 研究表明HCV表达的核心蛋白可以与HCV的核糖体插入位点(IRES)相互结合, 下调HCV病毒蛋白表达, 自我调控HCV复制, 降低HCV水平, 导致HCV在血液中间断性出现, 逃避机体免疫监视, 造成患者体内持续病毒感染[26-29]. 另外研究表明, HCV基因组上存在的IRES序列, 具有很强的翻译功能, 为双顺反子载体中效率较高的启动蛋白质翻译序列, 已经用于载体构建之中[30-33].
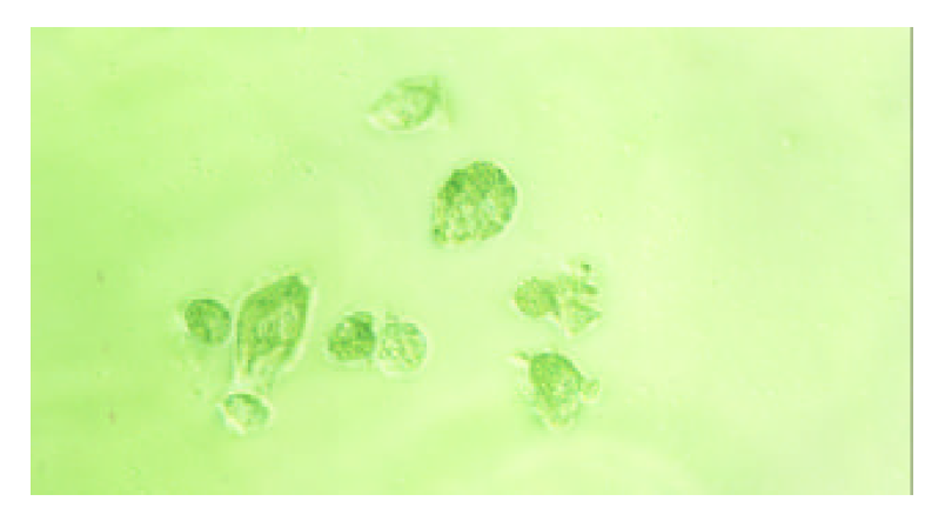
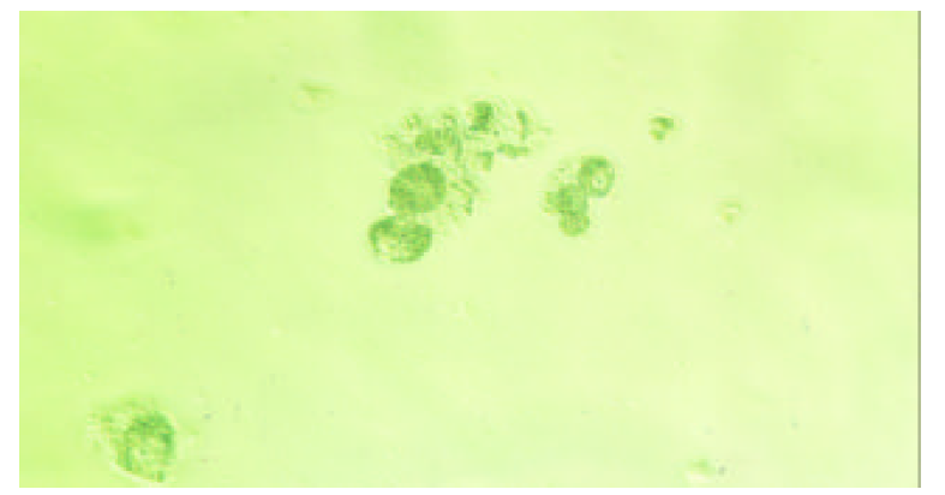
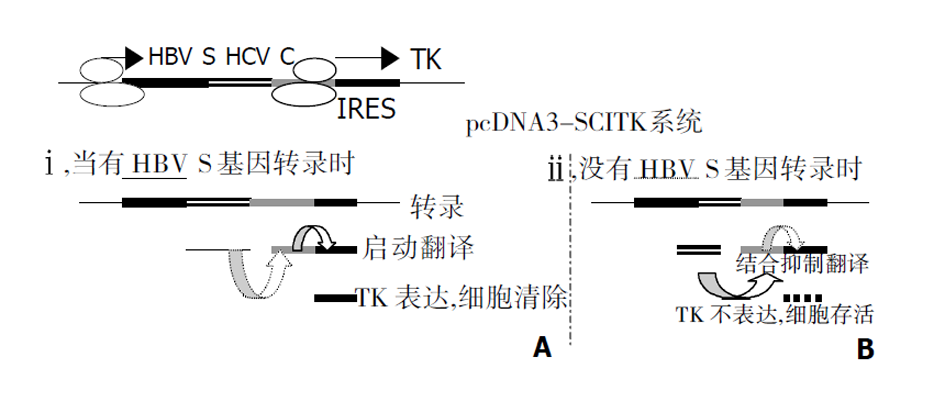
我们根据上述HCV复制特点, 在真核细胞表达载体pcDNA3的CMV启动子下依次驱动部分HBV S反义基因、HCV核心蛋白基因、HCV IRES部分有效序列和TK基因, 即HBV-anti S/HCV/core-IRES-TK载体系统, 构成一个以HBV 特有的S基因为靶向的基因功能相互平衡制约系统, 简称为SCITK系统, 当细胞内HBV病毒表达HBsAg, 这时S基因的mRNA即与载体系统中的S基因反义序列转录mRNA的相结合, 也就在HCV 核心蛋白基因起始密码子AUG前形成mRNA的二聚体, 抑制的HCV 核心蛋白mRNA翻译, HCV核心蛋白表达水平下降. HCV IRES结合蛋白减少, IRES启动下游基因TK表达相对增强, 受感染的肝细胞因表达HBsAg而自我同时表达TK, 肝细胞局部存在相对高浓度的TK, 而发挥TK溶解细胞效应, 细胞出现坏死和凋亡(图7A). ii, 在正常细胞内, 没有HBV S基因存在时, 表达的HCV核心蛋白与HCV IRES结合, 抑制IRES对下游TK的启动表达, 从而对机体细胞无害. 如果感染细胞HBV清除后, SCITK载体系统表达的TK逐渐降低和消失, 具有良好的调控性, 图7B.
为了验证上述理论, 我们构建了pcDNA3-SCI 和pcDNA3-SCITK载体. PCR和测序(图2-4)鉴定表明, 我们已经成功构建了一个以HBV S基因为靶向基因相互平衡制约系统. 将上述两种载体分别转染 2.2.15在转染后的0, 3, 6 d, 分别检测细胞培养基中HBsAg的表达量, 发现实验组中细胞分泌HBsAg呈波浪性, 而对照组细胞没有; 如果将pcDNA3-SCITK 载体分别转染2.2.15 细胞和HepG2细胞, 细胞凋亡明显增多, 细胞形态向凋亡方向发展(图5和图6). 该系统构建成功, 将为我们下一步的动物模型[34-36]研究奠定了坚实的基础.
| 1. | Chu CM, Liaw YF. Natural history of chronic hepatitis B virus infection: an immunopaththological study. J Gastroenterol. 1997;13:218. [DOI] |
| 2. | Rabe C, Pilz T, Klostermann C, Berna M, Schild HH, Sauerbruch T, Caselmann WH. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:208-215. [PubMed] [DOI] |
| 3. | Fang JN, Jin CJ, Cui LH, Quan ZY, Choi BY, Ki M, Park HB. A comparative study on serologic profiles of virus hepatitis B. World J Gastroenterol. 2001;7:107-110. [DOI] |
| 4. | Roussos A, Goritsas C, Pappas T, Spanaki M, Papadaki P, Ferti A. Prevalence of hepatitis B and C markers among refugees in Athens. World J Gastroenterol. 2003;9:993-995. [PubMed] [DOI] |
| 5. | Gao Y, Ma Y, Li M, Cheng T, Li SW, Zhang J, Xia NS. Oral immunization of animals with transgenic cherry tomatillo expressing HBsAg. World J Gastroenterol. 2003;9:996-1002. [PubMed] [DOI] |
| 6. | Wu CH, Ou-Yang EC, Walton C, Promrat K, Forouhar F, Wu GY. Hepatitis B virus infection of transplanted human hepatocytes causes a biochemical and histological hepatitis in immunocopetentent rats. World J Gastroenterol. 2003;9:978-983. [DOI] |
| 7. | Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23:47-58. [PubMed] [DOI] |
| 8. | Iino S. Natural history of hepatitis B and C virus infections. Oncology. 2002;62:18-23. [PubMed] [DOI] |
| 9. | Lau GK, Lai CL, Wu PC. The natural history of chronic hepatitis B infection. Hong Kong Med J. 1997;3:283-288. [PubMed] |
| 10. | Liaw YF. Therapy of chronic hepatitis B: current challenges and opportunities. J Viral Hepat. 2002;9:393-399. [DOI] |
| 11. | Chin R, Locarnini S. Treatment of chronic hepatitis B: current challenges and future directions. Rev Med Virol. 2003;13:255-272. [PubMed] [DOI] |
| 12. | Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61-68. [DOI] |
| 13. | Manns MP. Current state of interferon therapy in the treatment of chronic hepatitis B. Semin Liver Dis. 2002;22:7-13. [PubMed] [DOI] |
| 14. | Leung N. Treatment of chronic hepatitis B: case selection and duration of therapy. J Gastroenterol Hepatol. 2002;17:409-414. [DOI] |
| 15. | Di Bisceglie AM. Long-term outcome of interferon-a therapy for chronic hepatitis B. J Hepatol. 1995;22:s65-66. |
| 16. | Sokal E. Lamivudine for the treatment of chronic hepatitis B. Expert Opin Pharmacother. 2002;3:329-339. [PubMed] |
| 17. | Schiff ER. Lamivudine for hepatitis B in clinical practice. J Med Virol. 2000;61:386-391. [DOI] |
| 18. | Chayama K, Suzuki Y, Kobayashi M, Kobayashi M, Tsubota A, Hashimoto M, Miyano Y, Koike H, Kobayashi M, Koida I. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998;27:1711-1716. [PubMed] [DOI] |
| 19. | Romano G, Pacilio C, Giordano A. Genetransfer Technology in therapy: current applications and future goals. Stem Cell. 1999;17:191-202. [PubMed] [DOI] |
| 20. | Ferry N, Heard JM. Liver-directed gene transfer vectors. Hum Gene Ther. 1998;9:1795-1981. [PubMed] [DOI] |
| 21. | Dancer A, Julien S, Bouillot S, Pointu H, Vernet M, Huber P. Expression of thymidinekinase driven by an endothelial-specific promoter inhibits tumor growth of Lewis lung carcinoma cellsin transgenic mice. Gene Ther. 2003;10:1170-1178. [PubMed] [DOI] |
| 22. | Freund CT, Tong XW, Rowley D, Engehausen D, Frolov A, Kieback DG, Lerner SP. Combination of adenovirus-mediated thymidine kinase gene therapy with cytotoxic chemotherapy in bladder cancer in vitro. Urol Oncol. 2003;21:197-205. [DOI] |
| 23. | Kwon GY, Jeong J, Woo JK, Choi HY, Lee MJ, Ko JK, Shim YH, Kim CW. Co-expression of bfl-1 enhances host response in the herpes simplex virus-thymidine kinase/ganciclovir gene therapy system. Biochem Biophys Res Commun. 2003;303:756-763. [DOI] |
| 24. | Litvinova E, Maury S, Boyer O, Bruel S, Benard L, Boisserie G, Klatzmann D, Cohen JL. Graft-versus-leukemia effect after suicide-gene-mediated control of graft-versus-host disease. Blood. 2002;100:2020-2025. [PubMed] [DOI] |
| 25. | Chen SH, Chen XHL, Wang YB, Kosai K, Finegold MJ, Rich SS, Woo SL. Combination gene therapy for liver metastasis of colon carcinoma. Proc Natl Acad Sci. 1995;92:2577-2581. [DOI] |
| 26. | Yao ZQ, Ray S, Eisen-Vandervelde A, Waggoner S, Hahn YS. Hepatitis C virus: immunosuppression by complement regulatory pathway. Viral Immunol. 2001;14:277-295. [PubMed] [DOI] |
| 27. | Shimoike T, Mimori S, Tani H, Matsuura Y, Miyamura T. Interaction of hepatitis c virus core protein with viral sense RNA and suppression of its translation. J Virol. 1999;73:9718-9725. [PubMed] |
| 28. | Zhang J, Yamada O, Yoshida H, Iwai T, Araki H. Autogenous translational inhibition of core protein: implication for switch from translation to RNA replication in hepatitis C virus. Virology. 2002;293:141-150. [PubMed] [DOI] |
| 29. | Li D, Takyar ST, Lott WB, Gowans EJ. Amino acids 1-20 of the hepatitis C virus (HCV) core protein specifically inhibit HCV IRES-dependent translation in HepG2 cells, and inhibit both HCV IRES- and cap-dependent translation in HuH7 and CV-1 cells. J Gen Virol. 2003;84:815-825. [PubMed] [DOI] |
| 30. | Urab M, Hasumi Y, Ogasawara Y, Matsushita T, Kamoshita N, Nomoto A, Colosi P, Kurtzman GJ, Tobita K, Ozawa K. A novel discistronic AAV vector using a short IRES segement derived from hepatitis c virus genome. Gene. 1997;200:157-162. [DOI] |
| 31. | Kruger M, Beger C, Li QX, Welch PJ, Tritz R, Leavitt M, Barber JR, Wong-Staal F. Identification of eIF2Bgamma and eIF2gamma as cofactors of hepatitis C virus internal ribosome entry site-mediated translation using a functional genomics approach. Proc Natl Acad Sci USA. 2000;97:8566-8571. [DOI] |
| 32. | Zhang H, Hanecak R, Brown-Driver V, Azad R, Conklin B, Fox MC, Anderson KP. Antisense oligonucleotide inhibition of hepatitis C virus (HCV) gene expression in livers of mice infected with an HCV-vaccinia virus recombinant. Antimicrob Agents Chemother. 1999;43:347-353. [DOI] |
| 33. | Liang XS, Lian JQ, Zhou YX, Nie QH, Hao CQ. A small yeast RNA inhibits HCV IRES mediated translation and inhibits replication of poliovirus in vivo. World J Gastroenterol. 2003;9:1008-1013. [DOI] |
| 34. | Zoulim F, Berthillon P, Guerhier FL, Seigneres B, Germon S, Pichoud C, Cheng YC, Trepo C. Animal models for the study of HBV infection and the evaluation of new anti-HBV strategies. J Gastroenterol Hepatol. 2002;17:460-463. [DOI] |
| 35. | Wang CY, Giambrone JJ, Smith BF. Detection of duck hepatitis B virus DNA on filter paper by PCR and SYBR green dye-based quantitative PCR. J Clin Microbiol. 2002;40:2584-2590. [DOI] |
| 36. | Le Guerhier F, Pichoud C, Jamard C, Guerret S, Chevallier M, Peyrol S, Hantz O, King I, Trepo C, Cheng YC. Antiviral activity of beta-L-2, 3-dideoxy-2, 3-didehydro- 5-fluorocytidine in woodchucks chronically infected with woodchuck hepatitis virus. Antimicrob Agents Chemother. 2001;45:1065-1077. [PubMed] [DOI] |