修回日期: 2002-10-20
接受日期: 2002-11-18
在线出版日期: 2003-10-15
观察急性胰腺炎大鼠肝脏NF-κB对ICAM-1表达的调控及其在急性胰腺炎肝损伤中的作用.
Wistar大鼠72只随机分为急性胰腺炎组(AP组)、急性胰腺PDTC处理组(APP组)以及对照组(SO组). 分别在术后3 h、6 h、12 h及24 h检测肝组织NF-κB活性、ICAM-1表达, 肝组织髓过氧化物酶(MPO)活性以及血浆丙氨酸氨基转移酶(ALT)水平.
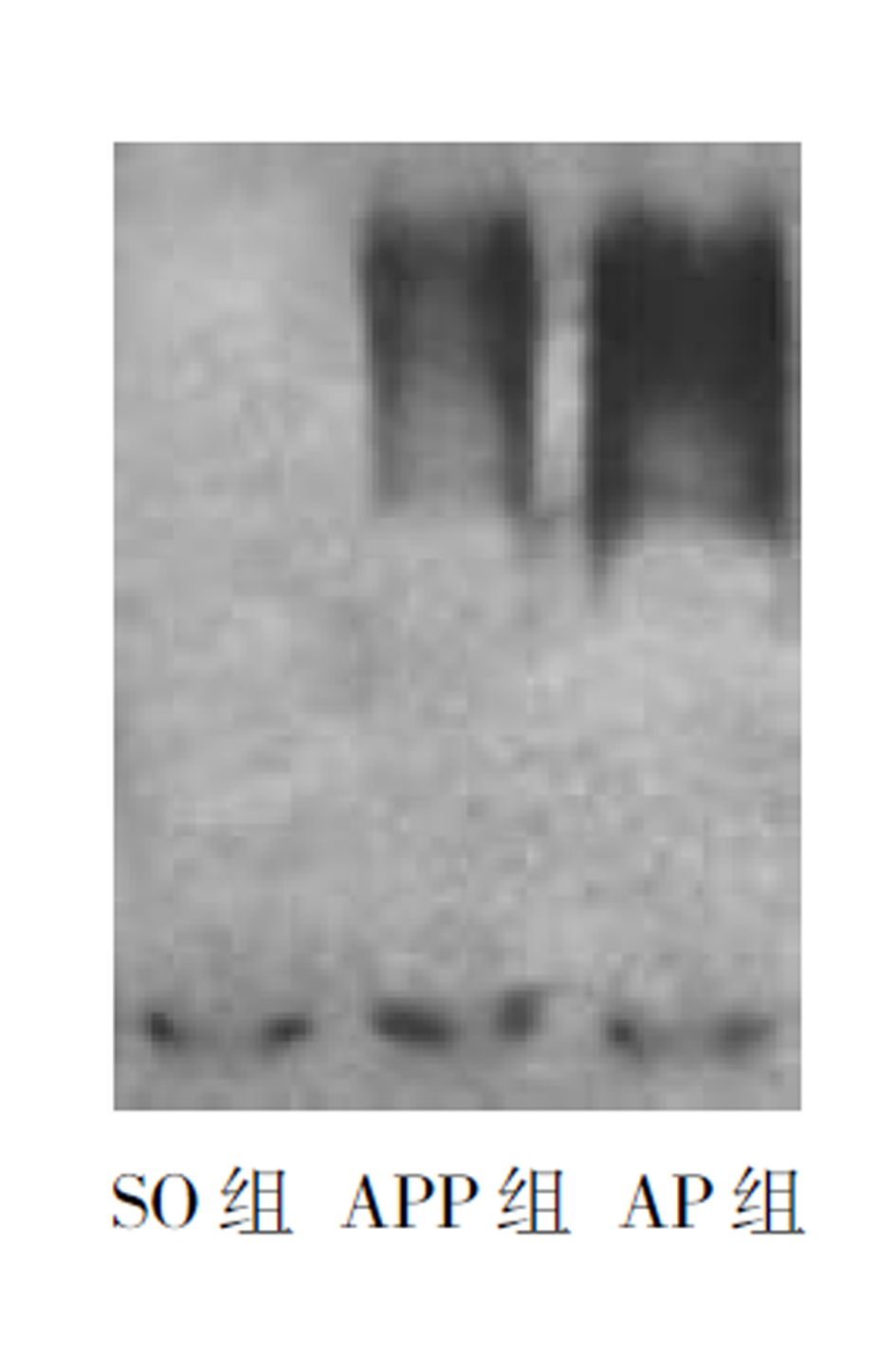
AP及APP组NF-κB活性在术后3-6 h显著高于SO组; ICAM-1表达在术后3-24 h显著高于SO组; MPO及ALT在术后6-24 h也显著高于SO组. 然而, 在运用了NF-κB抑制剂的APP组, NF-κB活性、ICAM-1表达、MPO以及血浆ALT均显著低于AP组.
急性胰腺炎发生时, 肝脏中活化的NF-κB促进了ICAM-1的表达, 并由此参与了肝损伤的发生.
引文著录: 石力, 田伏洲, 黄大熔, 李旭, 赵碧, 顾大勇, 唐旭东, 王雨. 急性胰腺炎大鼠肝脏NF-κB对ICAM-1表达的调控及其意义. 世界华人消化杂志 2003; 11(10): 1505-1507
Revised: October 20, 2002
Accepted: November 18, 2002
Published online: October 15, 2003
To study the effect of hepatic NF-κB on ICAM-1 expression in rats with acute pancreatitis.
Seventy-two Wistar rats were randomly divided into three groups: acute pancreatitis group (AP), acute pancreatitis treated with pyrrolidine dithiocarbamate(PDTC) group (APP) and sham operation group (SO). Hepatic NF-κB activities were determined with EMSA. The expression of hepatic ICAM-1 was detected with immunohistochemistry. Hepatic myeloperoxidase (MPO) and serum alanine aminotransferase (ALT) were measured.
Activities of NF-κB were significantly higher in AP and APP groups than that in SO group from 3 to 6 hours. The expressions of ICAM-1 were stronger in AP and APP groups than in SO group. The levels of hepatic MPO and serum ALT were also significantly higher in these two groups than in SO group. However, compared with AP group, the activities of NF-κB, the expression of ICAM-1 and the level of hepatic MPO and ALT significantly decreased in APP group.
Activation of hepatic NF-κB is involved in the liver injury by regulating ICAM-1 expression during pancreatitis.
- Citation: Shi L, Tian FZ, Huang DR, Li X, Zhao B, Gu DY, Tang XD, Wang Y. Effect of hepatic NF-κB on ICAM-1 expression in rats with acute pancreatitis. Shijie Huaren Xiaohua Zazhi 2003; 11(10): 1505-1507
- URL: https://www.wjgnet.com/1009-3079/full/v11/i10/1505.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i10.1505
急性胰腺炎常伴有多脏器功能不全[1], 而肝脏是最常受累的器官之一[2]. 核因子kB(NF-κB)是一类主要参与炎性分子表达调控的转录因子[3-5], 同参与其他原因引起的组织损伤过程一样, 他参与了急性胰腺炎肝损伤的发生[7-10]. 细胞间黏附分子-1(ICAM-1)主要介导多形核粒细胞(PMN)同血管内皮细胞之间的黏附, 他同样参与了不同原因引起的组织损伤过程[11,12]. 由于ICAM-1的启动子上存在着κB结合位点[13], 为了了解NF-κB参与急性胰腺炎肝损伤的机制, 我们观察了大鼠急性胰腺炎发生时, 肝脏NF-κB对ICAM-1表达的调节作用.
Wistar大鼠72只, 雌雄不限, 体质量200-250 g, 随机分为3组: (1)急性胰腺炎组(AP组, 24只): 用3 g/L戊巴比妥钠ip麻醉(10 mL/kg), 上腹部正中切口入腹, 经十二指肠穿刺胆管成功后, 以无创血管夹阻断肝门部胆管, 向胰管内逆行注入50 g/L牛磺胆酸钠(1 mL/kg), 注射完毕后松开血管夹, 关腹, 皮下注射生理盐水2 mL, 以补充术中丢失水分; (2)急性胰腺炎PDTC处理组(APP组, 24只): 诱导胰腺炎前1 h经腹腔注入NF-κB抑制剂PDTC (10 mg/kg); (3)假手术组(SO组, 24只): 操作方法同1, 但不向胰管内注入牛磺胆酸钠. 各组分别在术后3 h, 6 h, 12 h, 24 h再次开腹, 经下腔静脉采血约5 mL, 置于肝素钠抗凝瓶中, 离心(2000 r/min, 1 min), 取上清冻存; 并取大小约0.5 cm3肝中叶组织三块, 置于液氮中保存.
(1)肝组织NF-κB活性 参照文献介绍的方法对肝组织进行核蛋白提取, 并用考马斯亮蓝测定蛋白浓度, 调蛋白浓度为0.5 μg/L后置于-70°C保存. 以γ-32P在T4激酶的作用下标记NF-κB探针, 探针序列为5'-AGTGAGGGGACTTTCCCAGGC-3'. 将标记的探针同肝组织核蛋白进行结合后, 置于非变性聚丙稀酰胺凝胶中电泳并进行放射自显影, 用凝胶扫描分析仪对显影结果进行分析, 以积分辉度值表示NF-κB的活性变化. (2)肝组织ICAM-1蛋白表达 以免疫组化SP法进行检测, 用Tiger电子计算机图像分析系统计算阳性细胞染色的积分光密度. (3)肝组织髓过氧化物酶(MPO) 参照文献介绍的方法进行检测. (4)血浆丙氨酸氨基转移酶(ALT)用自动生化分析仪(Bckman CX7)进行检测.
统计学处理 结果以表示, 组间行t检验, P<0.05为差异有显著意义; P<0.01为差异有极显著意义.
除SO组外, 其余二组在术后3 h, 肝组织中NF-κB存在显著活化, 6 h后活化程度下降, 但仍显著高于SO组(P<0.01), 术后12 h NF-κB活化程度则明显下降, 同SO组相比无显著差异(P>0.05), 至24 h活化消失. 其中AP组NF-κB活化程度在3-6 h均显著高于APP组(P<0.01 表1, 图1).
| 项目 | 分组 | T (术后)/ h | |||
| 3 | 6 | 12 | 24 | ||
| NF-κB | AP | 3.13±0.57 | 1.92±0.26 | 0.80±0.12 | 0.68±0.17 |
| (吸光度) | APP | 1.85±0.38b | 1.36±0.22b | 0.73±0.11 | 0.71±0.13 |
| SO | 0.71±0.10d | 0.67±0.11d | 0.69±0.11 | 0.69±0.14 | |
| ICAM-1 | AP | 7.20±0.88 | 10.77±1.66 | 6.20±0.93 | 5.18±0.92 |
| (吸光度) | APP | 4.85±0.83b | 6.33±0.85b | 4.23±0.74b | 4.00±0.75a |
| SO | 1.88±0.24d | 1.45±0.36d | 1.57±0.31d | 1.45±0.33d | |
| MPO | AP | 2.13±0.45 | 4.51±0.78 | 6.45±0.93 | 11.40±1.62 |
| (kat/kg) | APP | 2.06±0.50 | 3.30±0.70a | 4.95±0.75a | 5.91±1.30b |
| SO | 1.71±0.41 | 1.82±0.38d | 1.76±0.50d | 1.56±0.37d | |
| ALT | AP | 1.09±0.19 | 1.56±0.21 | 2.37±0.36 | 3.47±0.73 |
| (kat/L) | APP | 0.96±0.14 | 1.17±0.18b | 1.65±0.21b | 2.13±0.43a |
| SO | 0.68±0.09 | 0.72±0.11d | 0.66±0.09d | 0.78±0.14d | |
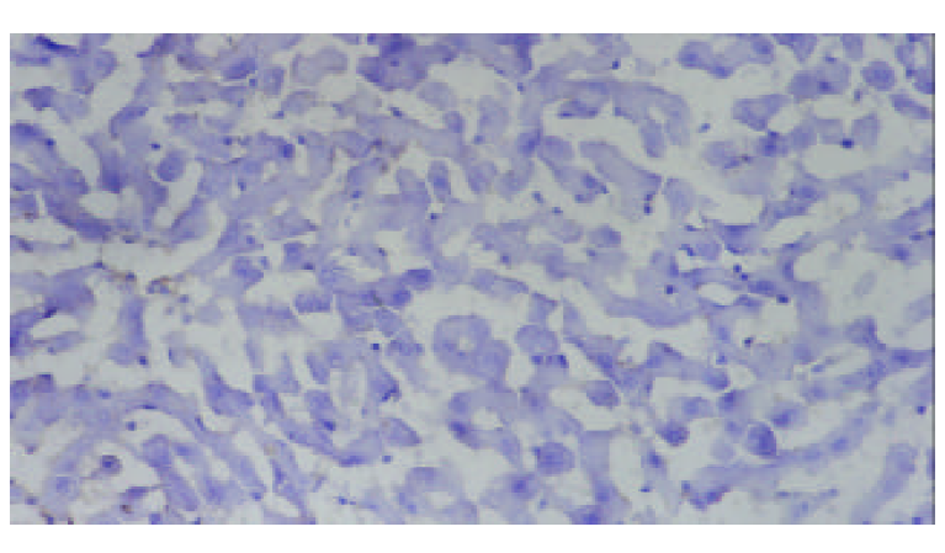
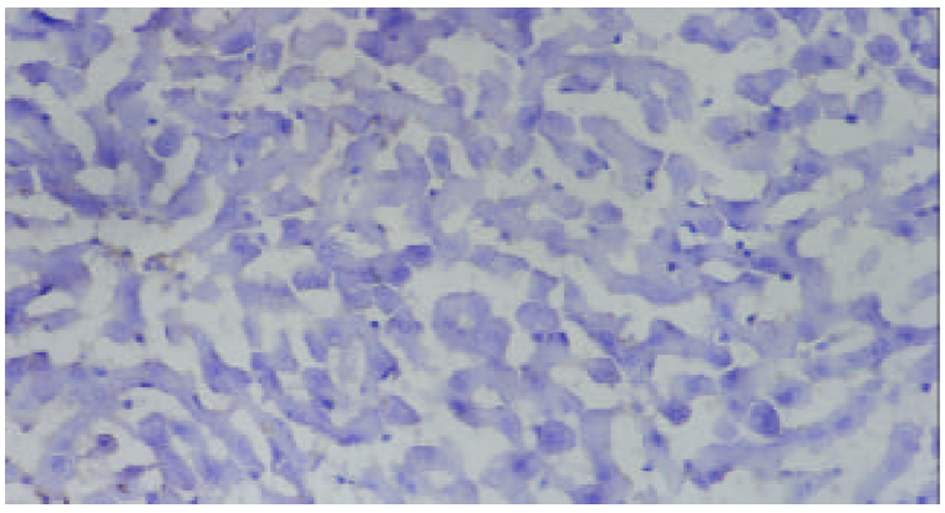
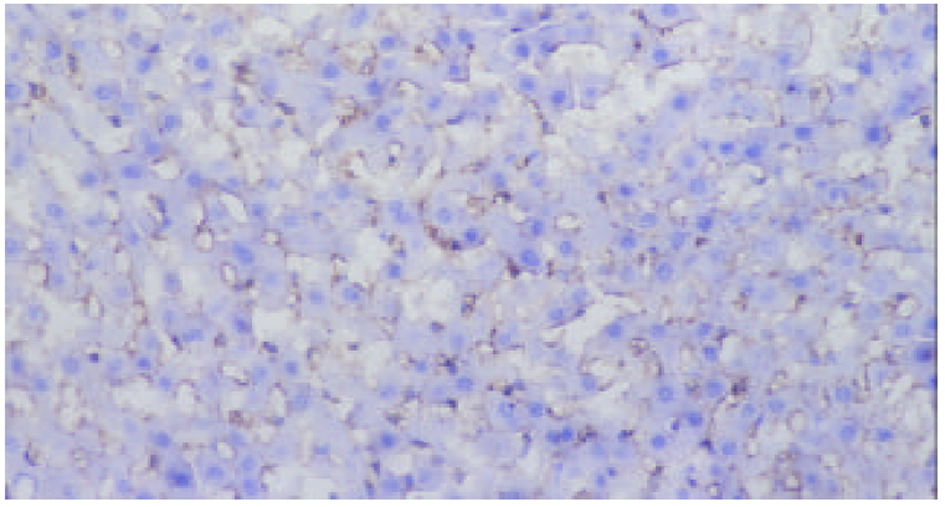
SO组未检测到ICAM-1蛋白表达, 在术后3-24 h, AP, APP组均有ICAM-1蛋白表达, 术后6 h表达最强, 各时相点均显著高于SO组(P<0.01). 阳性物质主要分布于肝窦内皮细胞及部分肝细胞膜. APP表达强度明显低于AP组(P<0.05或P<0.01, 表1、图2-4).
同SO组相比, AP、APP组MPO活性在术后6 h显著增加, 至24 h均呈持续性上升趋势(P<0.01). 其中APP组MPO活性在6-24 h均显著低于AP组 (P<0.05或P<0.01, 表1).
SO组ALT术后无明显变化. AP及APP组ALT在术后3-24 h均呈持续性上升, 显著高于SO组(P<0.01). 但6 h后APP组ALT明显低于AP组(P<0.05或P<0.01, 表1).
核因子κB是一类能与某些基因启动子及增强子区的κB序列结合的蛋白质, 他能启动或增强这些基因的转录[14-16]. 在静息的细胞中, NF-κB以无活性的形式存在于细胞质之中, 当受到一定的刺激后, NF-κB活化, 并发生核易位, 同靶基因启动子或增强子上的κB位点结合, 从而启动或增强这些基因的转录[17-20], 进而参与组织的损伤过程[22-24]. 在急性胰腺炎肝损伤发生过程中, 肝脏NF-κB的活化同样起着重要的作用[10].
由于在ICAM-1启动子上存在着NF-κB结合位点[13]. 为了进一步阐明NF-κB产生作用的机制, 我们观察了NF-κB对肝组织ICAM-1的表达的调控作用以及由此引起的组织损伤效应. ICAM-1是细胞黏附分子免疫球蛋白超家族中的一员, 主要介导多形核粒细胞(PMN)与血管内皮细胞间的黏附, 在PMN向组织中聚集的过程中有着重要的作用, 而聚积于组织中的PMN则可导致细胞及组织的损伤. 髓过氧化物酶(MPO)存在于PMN的嗜天青颗粒之中, 每个PMN所含MPO的量是恒定的, 因此通过测定MPO就能够反映PMN的浸润程度. 结果表明, 同对照组相比, 诱导急性胰腺炎后3-6 h, AP及APP组NF-κB出现了显著活化, 活化程度在3 h较高. 与此同时, ICAM-1蛋白表达在3-24 h也显著高于对照组, 其表达峰值出现在术后6 h, 较NF-κB有所滞后. 肝组织MPO及血浆ALT也显著高于对照组. 然而, 同AP组相比, 在运用了NF-κB抑制剂PDTC的APP组, 随着NF-κB活化程度的显著下降, ICAM-1蛋白表达、肝组织MPO以及血浆ALT在各时相点均有了显著下降. 这就说明在急性胰腺炎发生时, 肝脏NF-κB的活化促进了ICAM-1的表达, 并可由此导致PMN在肝组织中聚集, 从而引起肝损伤.
| 1. | Jin SL, Zhang TJ, Wang RY, Liao WJ, Song XW, Zhou YQ. The diagnosisi and treatment for the injury of heart, brain and lung in severe acute pancreatitis. Shijie Huaren Xiaohua Zazhi. 2001;9:1221-1223. |
| 2. | Shen J, Xiao H, Wu ZP, Song YH, Liu M. The role of arachidonic acid metabolites in liver damage in acute experimental pancreatitis in rats. Xin Xiaohuabingxue Zazhi. 1997;5:589-590. |
| 3. | Li YY, Gao ZF. Acute pancreatitis and NF-kB. Shijie Huaren Xiaohua Zazhi. 2001;9:420-421. |
| 4. | Suk K, Yeou Kim S, Kim H. Regulation of IL-18 production by IFN gamma and PGE2 in mouse microglial cells: involvement of NF-B pathway in the regulatory processes. Immunol Lett. 2001;77:79-85. [DOI] |
| 5. | Izumi T, Saito Y, Kishimoto I, Harada M, Kuwahara K, Hamanaka I, Takahashi N, Kawakami R, Li Y, Takemura G. Blockade of the natriuretic peptide receptor guanylyl cyclase-A inhibits NF-kappaB activation and alleviates myocardial ischemia/reperfusion injury. J Clin Invest. 2001;108:203-213. [PubMed] [DOI] |
| 6. | Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448-457. [DOI] |
| 7. | Breuss JM, Cejna M, Bergmeister H, Kadl A, Baumgartl G, Steurer S, Xu Z, Koshelnick Y, Lipp J, De Martin R. Activation of nuclear factor-kappa B significantly contributes to lumen loss in a rabbit iliac artery balloon angioplasty model. Circulation. 2002;105:633-638. [DOI] |
| 8. | Liu WB, Wang JY. NF-B and the apoptosis of hepatic ito cells. Shijie Huaren Xiaohua Zazhi. 2001;9:1054-1055. |
| 9. | Xu MQ, Xue L, Gong JP. Significance of kupffer cell NF-B activation during ischemia/reperfusion in rats. Shjie Huaren Xiaohua Zazhi. 2001;9:1250-1253. |
| 10. | Shi L, Huang DR, Tian FZ, Wang Y, Ma JX, Li XJ, Yin ZL. Relationship between NF-B activation and liver injuryin rats with acute pancreatitis. Shijie Huaren Xiaohua Zazhi. 2002;10:554-557. |
| 11. | Love S, Barber R. Expression of P-selectin and intercellular adhesion molecule-1 in human brain after focal infarction or cardiac arrest. Neuropathol Appl Neurobiol. 2001;27:465-473. [PubMed] [DOI] |
| 12. | Lo HP, Ackland-Berglund CE, Pritchard KA Jr, Guice KS, Oldham KT. Attenuated expression of inducible nitric oxide synthase in lung microvascular endothelial cells is associated with an increase in ICAM-1 expression. J Pediatr Surg. 2001;36:1136-1142. [PubMed] [DOI] |
| 13. | Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876-888. [PubMed] [DOI] |
| 14. | Antonelli A, Bianchi M, Crinelli R, Gentilini L, Magnani M. Modulation of ICAM-1 expression in ECV304 cells by macrophage-released cytokines. Blood Cells Mol Dis. 2001;27:978-991. [PubMed] [DOI] |
| 15. | Ginis I, Jaiswal R, Klimanis D, Liu J, Greenspon J, Hallenbeck JM. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J Cereb Blood Flow Metab. 2002;22:142-152. [PubMed] [DOI] |
| 16. | Wright G, Singh IS, Hasday JD, Farrance IK, Hall G, Cross AS, Rogers TB. Endotoxin stress-response in cardiomyocytes: NF-kappaB activation and tumor necrosis factor-alpha expression. Am J Physiol Heart Circ Physiol. 2002;282:H872-879. [PubMed] [DOI] |
| 17. | Lakshminarayanan V, Lewallen M, Frangogiannis NG, Evans AJ, Wedin KE, Michael LH, Entman ML. Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am J Pathol. 2001;159:1301-1311. [DOI] |
| 18. | Moine P, McIntyre R, Schwartz MD, Kaneko D, Shenkar R, Le Tulzo Y, Moore EE, Abraham E. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock. 2000;13:85-91. [DOI] |
| 19. | Valen G, Yan ZQ, Hansson GK. Nuclear factor kappa-B and the heart. J Am Coll Cardiol. 2001;38:307-314. [DOI] |
| 20. | Omoya T, Shimizu I, Zhou Y, Okamura Y, Inoue H, Lu G, Itonaga M, Honda H, NomuraM , Ito S. Effects of idoxifene and estradiol on NF-kappaB activation in cultured rat hepatocytes undergoing oxidative stress. Liver. 2001;21:183-191. [DOI] |
| 21. | Altavilla D, Saitta A, Guarini S, Galeano M, Squadrito G, Cucinotta D, Santamaria LB, Mazzeo AT, Campo GM, Ferlito M. Oxidative stress causes nuclear factor-kappaB activation in acute hypovolemic hemorrhagic shock. Free Radic Biol Med. 2001;30:1055-1066. [DOI] |
| 22. | Shames BD, Barton HH, Reznikov LL, Cairns CB, Banerjee A, Harken AH, Meng X. Ischemia alone is sufficient to induce TNF-alpha mRNA and peptide in the myocardium. Shock. 2002;17:114-119. [DOI] |
| 23. | Wang Z, Castresana MR, Detmer K, Newman WH. An IkappaB-alpha mutant inhibits cytokine gene expression and proliferation in human vascular smooth muscle cells. J Surg Res. 2002;102:198-206. [PubMed] [DOI] |
| 24. | Theuer J, Dechend R, Muller DN, Park JK, Fiebeler A, Barta P, Ganten D, Haller H, Dietz R, Luft FC. Angiotensin II induced inflammation in the kidney and in the heart of double transgenic rats. BMC Cardiovasc Disord. 2002;2:3. [PubMed] [DOI] |