修回日期: 2002-05-25
接受日期: 2002-06-19
在线出版日期: 2003-01-15
目的: 为研究肿瘤的多药耐药现象, 应用胃癌耐药相关的单克隆抗体MGr1筛选肿瘤耐药细胞表达文库获得的一个稳定阳性克隆的cDNA片段MGr1-Ag1, 检测MGr1-Ag1在胃癌组织中的分布及表达水平.
方法: 利用原位杂交技术, 对MGr1-Ag1 mRNA在胃癌组织中的分布进行了检测, 以了解其在胃癌中的表达情况及意义.
结果: MGr1-Ag1 mRNA定位在胃腺体细胞的胞质中, 为蓝色小颗粒, 呈弥漫性分布. MGr1-Ag1 mRNA在 SGC7901/VCR阳性信号明显高于SGC7901, 胃癌与癌旁组织相比, MGr1-Ag1 mRNA的阳性表达率均有显著差异(P<0.05). MGr1-Ag1 mRNA在胃癌(50.00%)、癌旁组织(71.42%)、高分化腺癌(68.75%)和中分化腺癌组织(57.14%)中的阳性表达率无显著差别, 但高分化组和中分化组都与低分化腺癌组织(10.00%)差别显著(P<0.05). 此外, MGr1-Ag1 mRNA还分布于小肠及大肠腺体内, 因例数偏少, 未做统计.
结论: MGr1-Ag1与胃癌的分化程度有关, 随着组织的分化程度增高, MGr1-Ag1 mRNA的阳性表达率增加.
引文著录: 尹芳, 时永全, 陈彩平, 乔泰东, 陈宝军, 苗继延, 樊代明. 胃癌耐药相关抗体MGr1筛选文库获得的基因MGr1-Ag1在胃癌组织中表达的研究. 世界华人消化杂志 2003; 11(1): 18-21
Revised: May 25, 2002
Accepted: June 19, 2002
Published online: January 15, 2003
AIM: To analyze the distribution and significance of the possible cDNA fragments encoding MGr1-Ag1 in human gastric cancer.
METHODS: We investigated MGr1-Ag1 expression in gastric cancer using in situ hybridization(ISH) techniques in 42 cases of gastric carcinomas in cryostatic sections collected from Xijing Hospital.
RESULTS: MGr1-Ag1 mRNA was positive in cytoplasm of gastric glandulous epithelia with intensive staining in 50.00% (21/42) cases with gastric cancer, and 71.42% (30/42) cases in paracancerous gastric tissues. Eleven of 16 (68.75%) cases were well differentiated, 8/14 (57.14%) cases were moderately differentiated and 1/10 (10.00%) cases were poorly differentiated. The positive rate of MGr1-Ag1 mRNA in gastric cancer was significantly different from that in paracancerous gastric tissues (71.42%). There was no significant difference in positive rate of MGr1-Ag1 mRNA among normal, paracancerous gastric tissues, well and moderately differentiated tissues, but the positive rate in the poorly differentiated tissues was low, being significantly different from that in well or moderately differentiated tissues.
CONCLUSION: MGr1-Ag1 mRNA is mainly localized in epithelial cells, and the level of its expression is correlated with the tumor differentiation.
- Citation: Yin F, Shi YQ, Chen CP, Qiao TD, Chen BJ, Miao JY, Fan DM. Expression of cDNA fragment encoding MGr1-Ag1 detected by MDR related antibody MGr1 in gastric cancer. Shijie Huaren Xiaohua Zazhi 2003; 11(1): 18-21
- URL: https://www.wjgnet.com/1009-3079/full/v11/i1/18.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i1.18
肿瘤多药耐药现象(multidrug resistance, MDR)是肿瘤治疗的热点和难点. 但目前的已知机制并不能充分解释肿瘤多药耐药性的发生, 而与耐药有关的分子和机制不断的被提出[1-9]. 为扩充肿瘤细胞产生MDR的机制, 以耐长春新碱人胃癌细胞SGC7901/VCR为免疫原制备了一株与胃癌耐药相关的单克隆抗体MGr1[10], 利用MGr1筛选肿瘤耐药细胞表位文库, 获得了一个稳定阳性克隆MGr1-Ag1, 携带长约262 bp的cDNA片段, 但其中218 bp的序列与定位于人染色体7p22-p21的部分基因组序列完全相同[11]. 为分析MGr1-Ag1片段可能的意义及功能, 利用mRNA分子原位杂交等技术, 对MGr1抗体筛选的cDNA片段MGr1-Ag1在胃癌的表达情况进行了检测.
42例胃癌组织及其癌旁组织取自第四军医大学西京医院于1999-08/1999-12间行胃癌切除术的患者, 正常胃黏膜标本25例取自胃镜活检. 所有标本均于离体后30 min内完成取材并置于液氮中保存. 制备8 mm冰冻切片, 40 g/L多聚甲醛固定、干燥, -70 ℃冰箱保存. 所有病理标本均经病理科两位以上医师诊断. 胃癌细胞SGC7901和胃癌耐长春新碱SGC7901/VCR为我所保存的细胞. 胰酶和小牛血清为Sigma公司产品. RPMI1640和BSA为Gibco BRL产品. 多聚赖氨酸胶购于武汉博士德公司. 限制内切酶、DNA纯化试剂盒及其他常规试剂购于华美生物工程公司. DIG DNA Labeling and Detection Kit购于德国Boehringer Mannheim公司.
1.2.1 组织切片准备: 培养细胞在多聚赖氨酸处理的盖玻片上进行培养, 培养液为80 ml/L小牛血清RPMI1640. 细胞长好后10 mol/L PBS(pH7.4)洗3×2 min. 细胞爬片和冰冻切片在40 g/L多聚甲醛室温固定10 min.10 mol/L PBS(pH7.4)洗3×2 min. 干燥后冰冻保存.
1.2.2 探针的制备和标记: MGr1-Ag1 cDNA片段克隆在pSPORT1载体SalⅠ和NotⅠ位点之间, 两侧有BamHⅠ和KpnⅠ的位点. BamHⅠ和KpnⅠ消化质粒. 将酶切产物进行10 g/L琼脂糖凝胶电泳, 得到约0.3 kb cDNA片段. 试剂盒纯化回收cDNA片段并定量. 取1-3 mg cDNA煮沸10 min, 冰水中冷却. 用地高辛DNA标记试剂盒进行标记和纯化.
1.2.3 mRNA: 原位杂交切片于70 ℃ 2×SSC预热15 min, PBS洗切片2 min. 切片上滴加0.5 mol/L TBS(pH7.2-7.6)稀释的1: 1 000 Proteinase K, 37 ℃消化2-15 min, 暴露mRNA核酸片段. 置4 ℃预冷的40 g/L多聚甲醛中平衡5 min, PBS洗2×2 min. 2×SSC洗2×2 min. 逐级乙醇脱水. 每张切片上加适量预杂交液, 湿盒70 ℃孵育8 min, 37 ℃孵育1 h. 将标记的cDNA探针放入95-100 ℃水中煮沸5-10 min后, 迅速冷却3 min. 按0.5-1.0 mg/mL在杂交液加入标记探针.37 ℃湿盒中杂交16-24 h后, 室温2×SSC洗20 min, 1×SSC洗20 min, 43 ℃ 0.5×SSC 洗20 min, 室温0.5×SSC洗20 min. BufferⅠ室温振洗1×5 min, 1×30 min, BufferⅢ作用5 min, 加抗地高辛抗体-AP复合物(1: 500), 37 ℃2h. BufferⅠ浸洗2×15 min, 振洗1×5 min, BufferⅢ浸洗5 min, 加显色液显色0.5-12 h. 染色理想时终止反应. 上述所用试剂和材料均经去RNA酶处理.
1.2.4 结果判定: 显微镜下选取染色结果较理想部位, 于高倍镜下, 每视野统计200个细胞数. 阳性细胞数比率<10%或无阳性着色为阴性(-); 阳性细胞数比率<50%为弱阳性(+); 阳性细胞数比率>50%为阳性(++); 阳性细胞数比率>75%为强阳性(+++).
统计学处理 采用SPSS9.0统计软件进行x2检验, 以P<0.05确定为有统计学意义.
用BamHⅠ和KpnⅠ酶消化pSPORT1质粒, 得到一长约0.3 kb片段, 携带262 bp 的MGr1-Ag1 cDNA片段.
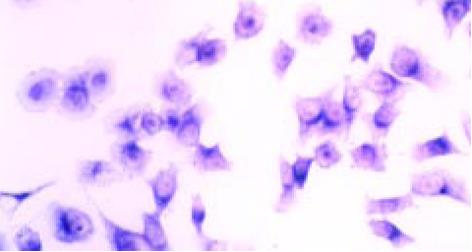
MGr1-Ag1 mRNA在胃癌耐药细胞系SGC7901/VCR中的表达强度明显高于SGC7901. MGr1-Ag1 mRNA定位在SGC7901/VCR和SGC7901细胞的胞质中, 为蓝色小颗粒, 呈弥漫性分布(图1, 图2). 在胃组织中, MGr1-Ag1的mRNA定位于的胃腺体细胞, 在胃癌组织中阳性率为50.00%; 在癌旁胃黏膜中的阳性表达率为71.42%; 在正常胃黏膜的阳性表达率为68.00%. 胃癌与癌旁组织相比, MGr1-Ag1 mRNA的阳性表达率有显著差异(P<0.05)(表1).
| Group | № | MGr1-Ag1 | T | R(%) | |||
| - | + | ++ | +++ | ||||
| GC | 42 | 21 | 9 | 10 | 2 | 21 | 50.00 |
| PC | 42 | 12 | 12 | 15 | 3 | 30 | 71.42 |
| NT | 25 | 8 | 5 | 9 | 3 | 17 | 68.00 |
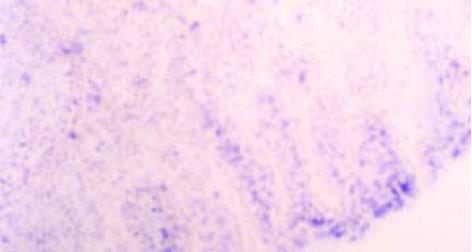
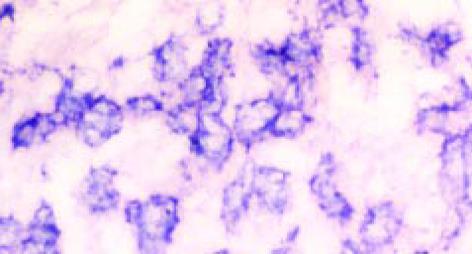
MGr1-Ag1 mRNA的表达水平在癌旁组织、正常胃黏膜组织、高分化腺癌(68.75%)和中分化腺癌(57.14%)的阳性表达率无显著差别(图3, 图4), 但高分化组和中分化组与低分化腺癌(10.00%)的阳性表达率差别显著(P<0.05)(表2). 此外, MGr1-Ag1 mRNA还分布于小肠及大肠腺体内, 因例数偏少, 未做统计.
| Group | № | Grade of intensity | T | R(%) | |||
| - | + | ++ | +++ | ||||
| WD | 16 | 5 | 5 | 5 | 1 | 11 | 68.75 |
| MD | 14 | 6 | 3 | 4 | 1 | 8 | 57.14 |
| PD | 10 | 9 | 1 | 0 | 0 | 1 | 10.00 |
| MC | 2 | 1 | 0 | 1 | 0 | 1 | 50.00 |
胃癌对化疗药物的反应性通常很差[12,13], 肿瘤细胞可通过各种途径产生对化疗的抵抗性, 其机制复杂多样. 肿瘤细胞能通过P-糖蛋白和MRP增加药物的外排[14-18] ; 位于胞质的MRP1和肺耐药蛋白能改变细胞内药物的分布[19-23] ; GST通过催化GSH与抗肿瘤药物结合增强细胞解毒功能[24-26] ; TopoⅡ发生改变, 可引起以其为靶点的药物诱导的DNA稳定断裂复合物形成减少, DNA双链断裂减少, 细胞产生耐药性[27,28]. 此外一些代谢酶类及涉及凋亡和信号转导的分子也参与耐药性的形成[29-33].
我所采用大剂量间歇诱导法以长春新碱诱导人胃癌细胞SGC7901, 获得了人胃癌耐药细胞株SGC7901/VCR, 该细胞具有MDR表型, 耐受5-氟尿嘧啶、顺铂、柔红霉素等多种结构不同的药物. 以SGC7901/VCR为免疫原, 采用杂交瘤技术制备了胃癌细胞耐药相关性单克隆抗体, 命名为MGr1[10]. MGr1抗原在SGC7901/VCR的胞膜上和胞质中表达, 且显著高于亲本细胞SGC7901. Western blot结果表明MGr1抗原分子量大约为40 KDa. MGr1抗体能部分逆转SGC7901/VCR对阿霉素、长春新碱和5-氟尿嘧啶的耐药性, 显著增加SGC7901/VCR细胞内阿霉素的潴留. 说明MGr1是肿瘤耐药相关性抗体. 为了克隆MGr1抗原的编码基因, 研究MGr1抗原的分子结构和功能, 利用MGr1抗体筛选肿瘤耐药细胞表达文库, 获得了一个稳定阳性克隆MGr1-Ag1. MGr1-Ag1 mRNA的表达水平在癌旁组织、正常胃黏膜组织、高分化腺癌和中分化腺癌的阳性表达率无显著差别, 但与低分化腺癌的阳性表达率差别显著(P<0.05), 提示MGr1-Ag1与胃癌的分化程度有关, 随着组织的分化程度增高, MGr1-Ag1 mRNA的阳性表达率也增加, 该结果与利用免疫组化和MGr1抗体检测MGr1-Ag在胃癌组织中的表达分布情况基本一致.
我们将继续用MGr1抗体筛选肿瘤cDNA文库, 采用RACE技术克隆MGr1-Ag1全长cDNA, 并通过基因转染分析MGr1-Ag1在胃癌MDR中的作用, 从而判断MGr1-Ag1是否与肿瘤细胞耐药现象有关, 并最终获得MGr1-Ag的编码基因, 为充实胃癌MDR机制和从基因水平寻找新的耐药逆转措施奠定良好的基础.
编辑: N/A
| 1. | Duesberg P, Stindl R, Hehlmann R. Origin of multidrug resistance in cells with and without multidrug resistance genes: chromosome reassortments catalyzed by aneuploidy. Proc Natl Acad Sci U S A. 2001;98:11283-11288. [PubMed] [DOI] |
| 2. | Um JH, Kang CD, Lee BG, Kim DW, Chung BS, Kim SH. Increased and correlated nuclear factor-kappa B and Ku autoantigen activities are associated with development of multidrug resistance. Oncogene. 2001;20:6048-6056. [PubMed] [DOI] |
| 3. | Yang JM, Vassil AD, Hait WN. Activation of phospholipase C induces the expression of the multidrug resistance (MDR1) gene through the Raf-MAPK pathway. Mol Pharmacol. 2001;60:674-680. [PubMed] |
| 4. | Ross DD. Novel mechanisms of drug resistance in leukemia. Leukemia. 2000;14:467-473. [PubMed] [DOI] |
| 5. | Baron JM, Höller D, Schiffer R, Frankenberg S, Neis M, Merk HF, Jugert FK. Expression of multiple cytochrome p450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J Invest Dermatol. 2001;116:541-548. [PubMed] [DOI] |
| 6. | Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54-59. [PubMed] [DOI] |
| 7. | Carobbio S, Realini C, Norbury CJ, Toda T, Cavalli F, Spataro V. Sequence of Crm1/exportin 1 mutant alleles reveals critical sites associated with multidrug resistance. Curr Genet. 2001;39:2-9. [PubMed] [DOI] |
| 8. | Lage H, Perlitz C, Abele R, Tampé R, Dietel M, Schadendorf D, Sinha P. Enhanced expression of human ABC-transporter tap is associated with cellular resistance to mitoxantrone. FEBS Lett. 2001;503:179-184. [PubMed] [DOI] |
| 9. | Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2). J Cell Sci. 2000;113:2011-2021. [PubMed] |
| 11. | 时 永全, 肖 冰, 苗 继延, 李 明峰, 乔 泰东, 陈 宝军, 陈 峥, 韩 军良, 周 绍娟, 樊 代明. MGr1抗体筛选文库获得胃癌耐药相关的新基因片段. 华人消化杂志. 1998;6:656-669. [DOI] |
| 12. | Alexander D, Yamamoto T, Kato S, Kasai S. Histopathological assessment of multidrug resistance in gastric cancer: expression of P-glycoprotein, multidrug resistance-associated protein, and lung-resistance protein. Surg Today. 1999;29:401-406. [PubMed] [DOI] |
| 13. | Endo K, Maehara Y, Kusumoto T, Ichiyoshi Y, Kuwano M, Sugimachi K. Expression of multidrug-resistance-associated protein (MRP) and chemosensitivity in human gastric cancer. Int J Cancer. 1996;68:372-377. [PubMed] [DOI] |
| 14. | Grandjean F, Brémaud L, Verdier M, Robert J, Ratinaud MH. Sequential gene expression of P-glycoprotein (P-gp), multidrug resistance-associated protein (MRP) and lung resistance protein: functional activity of P-gp and MRP present in the doxorubicin-resistant human K562 cell lines. Anticancer Drugs. 2001;12:247-258. [PubMed] [DOI] |
| 15. | Kong XB, Yang ZK, Liang LJ, Huang JF, Lin HL. Overexpression of P-glycoprotein in hepatocellular carcinoma and its clinical implication. World J Gastroenterol. 2000;6:134-135. [PubMed] [DOI] |
| 16. | van der Kolk DM, de Vries EG, Noordhoek L, van den Berg E, van der Pol MA, Müller M, Vellenga E. Activity and expression of the multidrug resistance proteins P-glycoprotein, MRP1, MRP2, MRP3 and MRP5 in de novo and relapsed acute myeloid leukemia. Leukemia. 2001;15:1544-1553. [PubMed] [DOI] |
| 17. | Bagrij T, Klokouzas A, Hladky SB, Barrand MA. Influences of glutathione on anionic substrate efflux in tumour cells expressing the multidrug resistance-associated protein, MRP1. Biochem Pharmacol. 2001;62:199-206. [PubMed] [DOI] |
| 18. | Young LC, Campling BG, Cole SP, Deeley RG, Gerlach JH. Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: correlation of protein levels with drug response and messenger RNA levels. Clin Cancer Res. 2001;7:1798-1804. [PubMed] |
| 19. | Han K, Kahng J, Kim M, Lim J, Kim Y, Cho B, Kim HK, Min WS, Kim CC, Lee KY. Expression of functional markers in acute nonlymphoblastic leukemia. Acta Haematol. 2000;104:174-180. [PubMed] [DOI] |
| 20. | Coley HM. Drug resistance studies using fresh human ovarian carcinoma and soft tissue sarcoma samples. Keio J Med. 1997;46:142-147. [PubMed] [DOI] |
| 21. | Rimsza LM, Campbell K, Dalton WS, Salmon S, Willcox G, Grogan TM. The major vault protein (MVP), a new multidrug resistance associated protein, is frequently expressed in multiple myeloma. Leuk Lymphoma. 1999;34:315-324. [PubMed] [DOI] |
| 22. | Siva AC, Raval-Fernandes S, Stephen AG, LaFemina MJ, Scheper RJ, Kickhoefer VA, Rome LH. Up-regulation of vaults may be necessary but not sufficient for multidrug resistance. Int J Cancer. 2001;92:195-202. [PubMed] [DOI] |
| 23. | Liu ZM, Shou NH, Jiang XH. Expression of lung resistance protein in patients with gastric carcinoma and its clinical significance. World J Gastroenterol. 2000;6:433-434. [PubMed] [DOI] |