Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1374
Revised: January 1, 2003
Accepted: January 8, 2003
Published online: June 15, 2003
AIM: This retrospective study was designed to analyze the results and the failure patterns of late course accelerated hyperfractionated radiotherapy for clinical T1-2N0M0 esophageal carcinoma.
METHODS: From Aug. 1994 to Feb. 2001, 56 patients with clinical T1-2 esophageal carcinoma received late course accelerated hyperfractionated radiotherapy in Cancer Hospital, Fudan University. All patients had been histologically proven to have squamous cell carcinoma (SCC) and were diagnosed to be T1-2N0M0 by CT scan. All patients were treated with conventional fractionation (CF) irradiation during the first two-thirds course of the treatment to a dose of about 41.4Gy/23fx/4 to 5 wk, Which was then followed by accelerated hyperfractionation irradiation using reduced fields, twice daily at 1.5Gy per fraction, to a dose about 27Gy/18 fx. Thus the total dose was 67-70Gy/40-43fx/40-49 d.
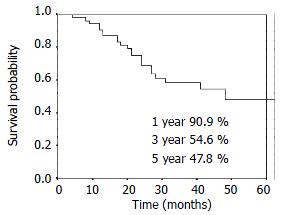
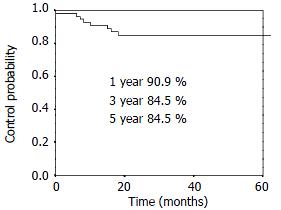
RESULTS: The 1-, 3- and 5-year overall survival was 90.9%, 54.6%, 47.8% respectively. The 1-, 3- and 5-year local control rate was 90.9%, 84.5% and 84.5%, respectively. Twenty-five percent (14/56) patients had distant metastasis and/or lymph nodes metastasis alone. Eight point nine percent (5/56) patients had local disease alone. Another 3.6% (2/56) patients had regional relapse and distant metastasis.
CONCLUSION: Late course accelerated hyperfractionated radiotherapy is effective on clinical T1-2 esophageal carcinoma. The main failure pattern is distant metastasis.
- Citation: Zhao KL, Wang Y, Shi XH. Late course accelerated hyperfractionated radiotherapy for clinical T1-2 esophageal carcinoma. World J Gastroenterol 2003; 9(6): 1374-1376
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1374.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1374
Surgery has been the main treatment method for clinical T1-2 esophageal carcinoma. But the treatment of upper thoracic esophageal carcinoma is challenging. The intimate relationship of the esophagus to the airway, arch of the aorta, and recurrent laryngeal nerve poses special technical problems. Radiotherapy is as effective as surgery, and preserves esophagus.
In 1988, Shi designed the schedule of late course accelerated hyperfractionated radiotherapy (LCAF) on SCC of the esophagus. The results were very encouraging. The 5-year survival and local control rate were markedly improved in the LCAF group. Compared with CF radiotherapy, the 5-year overall survival of 34% versus 15% was statistically significant, the local control rate was 55% versus 21%[1]. However, the outcome of clinical T1-2 esophageal carcinoma treated with LCAF has not been investigated extensively. Therefore, we conducted a retrospective evaluation of clinical T1-2 patients treated with LCAF.
From August 1994 to February 2001, 56 patients with clinical T1-2N0M0 SCC of esophagus were treated by LCAF radiotherapy in the Department of Radiation Oncology, Cancer Hospital, Fudan University. All patients had detailed medical records. Pretreatment evaluation generally included history and physical examination, complete blood cell count, chest radiograph, chest computed tomographic (CT) scan, esophageal barium examination, ultrasonic examination for abdomen, including liver, kidney, spleen, and retroperitoneal lymph nodes. All patients were restaged according to the TNM classification of the International Union Against Cancer (devised in 1997). The patients eligibility for this study was as follows: (1) Primary lesion was single, and a flat plane separating the esophageal mass from the periesophageal structures was visible on all CT sections. (2) Mediastinal and upper abdominal lymph nodes were smaller than 5 mm on short-axis diameter. And (3) No supraclavicular lymph nodes and distant metastasis. The patients clinical characteristics are listed in Table 1.
| Characteristic | n | % |
| Age(years) | ||
| < 65 | 30 | 53.6 |
| ≥ 65 | 26 | 46.4 |
| Median | 59.5 | |
| Range | 40-74 | |
| Gender | ||
| Male | 41 | 73.2 |
| Female | 15 | 26.8 |
| T stage | ||
| T1 | 2 | 3.7 |
| T2 | 54 | 96.3 |
| Site | ||
| Cervical | 2 | 3.6 |
| Upper-thoracic | 19 | 33.9 |
| Middle-thoracic | 34 | 60.7 |
| Lower-thoracic | 1 | 1.8 |
| Length (cm) | ||
| ≤ 5 | 25 | 44.6 |
| > 5 | 31 | 55.4 |
| Median | 5.8 | |
| Range | 2.0-9.0 | |
| Thickness of wall (cm) | ||
| ≤ 1.5 | 42 | 75.0 |
| > 1.5 | 14 | 25 |
| Median | 1.2 | |
| Range | 0.5-2.7 | |
Radiation source was 6MV or 18 MV linear accelerator. The design of the radiation fields was based on the diagnosis by CT and barium examinations. For patients with lesions in cervical region, two anterior oblique fields with wedge filters were used. For patients with lesions in the thoracic a three-field approach was used: one anterior and two posterior oblique portals. The width of the fields was adjusted to cover gross tumor with 2 cm to 3 cm margins to include the subclinical lesions and the length of the field should cover clinical tumors with 3 cm to 5 cm extended margin at both ends of the lesion. All patients received 1.8Gy per fraction, five fractions a week during the first two thirds of the course of radiotherapy to a dose of about 41.4Gy/23fx/4 to 5 wk. This was then followed by accelerated hyperfractionation using reducing fields, twice daily at 1.5Gy per fraction with a minimum interval of 6 h between fractions. The dose contributed by the accelerated technique was about 27Gy. The total dose given to the clinical tumor was 67-70Gy/40-43fx/40-49d. No prophylactic irradiation was given to the supraclavicular region. Details of the schedule had been reported previously[1].
End-points in this analysis were overall survival and local control. Death from any cause was calculated from the date of radiotherapy until death or last follow-up evaluation. Patterns of failure were first failure (local, regional, or distant), time to any local failure, and time to any distant metastasis. If recurrences occurred within 60 d of each other, they were counted simultaneously. The time to these end points was calculated from the date of treatment until disease relapse or last follow-up.
Four patients were lost to follow-up. Median follow-up for the survival patients was 38.0 mo (range 5-67 mo), follow-up rate was 92.9%.
The statistics was done by SPSS (Version 10.0). Survival rate and local control rate were estimated by the Kaplan-Meier method.
The incidence of grade 1, 2, 3 and 4 of acute radiation-induced bronchitis was 19.6% (11/56), 17.9% (10/56), 3.6% (2/56), and 1.8% (1/56), respectively. The incidence of grade 1, 2, 3 and 4 of acute radiation-induced esophagitis was 25.0% (14/56), 46.4% (26/56), 10.7% (6/56), and 0% (0 cases), respectively.
Three patients (53.6%) developed grade 2 radiation-induced late esophageal stenosis, and one patient (1.8%) developed grade 3 esophageal stenosis (the patient had required dilatation). One patient (1.8%) developed grade 2 late pneumonary fibrosis. Two patients (3.6%) died of late complications (1 case died of pneumonary fibrosis, 1 case died of myelitis).
The overall survival curve and the local control rates for all patients are shown in Figure 1, Figure 2. The overall survival rate at 1-, 3- and 5-year was 90.9%, 54.6% and 47.8%, respectively. The local control rate at 1-, 3- and 5-years was 90.9%, 84.5% and 84.5%, respectively.
The patterns of first failure are listed in Table 2. The main first failures were local regional failure and distant metastasis (including lymph metastasis). Twenty-five percent (14/56) patients had distant metastasis and/or lymph nodes metastasis alone. Eight point nine percent (5/56) had local disease alone. Another 3.6% (2/56) had local regional relapse and distant metastasis. The median local regional failure time was 9 mo (rang: 0-18 mo). The median distant metastasis time was 17.5 mo (rang: 7-65 mo).
| First failure | n | % |
| None | 31 | 55.4 |
| Local/regional only | 5 | 8.9 |
| Distant only | 14 | 25.0 |
| Local/regional/distant | 2 | 3.6 |
| Hemorrhage | 1 | 1.8 |
| Complications | 2 | 3.6 |
| Unknown | 1 | 1.8 |
| Total | 56 | 100 |
Surgery is the main treatment for clinical T1-2 esophageal carcinoma. In a nationwide surgical series from 1983 to 1987 in Japan[2], the survival rate of 127 patients with mucosal tumors was 92.26% at 1 year, 81.09% at 3 years, and 89.85% at 5 years. A total of 533 patients with submucosal tumor had survival rate of 83.51% at 1 year, 66.76% at 3 years, and 59.66% at 5 years. T1 classification by UICC (1987) combines mucosal and submucosal tumors. Seven hundred forty patients with invasion to the muscularis propria, equal to T2 by UICC classification, had survival rate of 69.86% at 1 year, 41.88% at 3 years, and 34.47% at 5 years. Because a nearly total esophagectomy is often required, higher mortality and morbidity occur often. Orringer[3] reported 800 patients with cancer of the intrathoracic esophagus and cardia treated with transhiatal esophagectomy. Major complications included anastomotic leaks (13%), recurrent laryngeal nerve injury (7%), wound infection (3%), pulmonary complications (2%), bleeding (1%), and chylothorax (1%). More than 90% of patients were discharged within 21 d of hospitalization. Complications were more severe in the patients with carcinoma of upper thoracic esophagus. The operation of upper thoracic esophageal cancer is challenging. The intimate relationship of the esophagus to the airway, arch of the aorta, and recurrent laryngeal nerve poses special technical problems. The survival rate is often lower, and the mortality and morbidity are often higher. Vigneswaran[4] reported 49 patients treated with extended esophagectomy. There was one postoperative death (2.0%). Fifteen patients (30.6%) had a cervical anastomotic leak, and all occurred in patients with a gastric conduit. Leaks occurred in 5 of 10 (50%) substernally placed stomachs and in 10 of 36 (27.7%) transposed through the esophageal bed. Wound infection occurred in five patients. Postoperative vocal cord paralysis developed in 11 patients (22.4%) and was unilateral in 10 and bilateral in one. Six patients developed pulmonary complications. Ten patients (20.8%) developed mild to severe late dysphagia, which was a result of benign stenosis in eight patients and recurrent carcinoma in two. Four patients required dilatations.
Chemoradiotherapy has been widely used in Western countries. Reports on definitive chemoradiotherapy for clinical T1-2 esophageal carcinoma are few. Zenone et al[5] concluded that the combined multimodality therapy, might be an alternative to radical surgery, based on 5-year survival of 56.3% for T1 patients and 29.8% for T2 patients. Roca et al[7] also suggested the feasibility of organ preservation in a report using intensive chemoradiotherapy. Murakami et al[2] reported in a randomized study comparing chemoradiation with surgery that overall 1- and 3-year survival rates were 100% and 83% in the T1/protocol group versus 82% and 72% in the T1/surgery group (P = 0.36), and 100% and 51% in the T2/protocol group, versus 95% and 68% in the T2/surgery group (P = 0.61), respectively. There was no treatment-related mortality in either group. The rate of esophageal conservation was 92% in the T1/protocol group and 58% in the T2/protocol group[2]. These results indicate that chemoradiation for clinical T1-2 patients is a feasible radical treatment. However, there has been no report that compares surgery and chemoradiotherapy. Although there was a significant improvement in local control and overall survival with combined multimodality therapy compared with radiation therapy alone, the combined treatment had more severe and life-threatening hematologic side effects. Grade 3-5 acute toxicity was seen in 64% of patients treated by concurrent chemoradiotherapy, much higher than 28% in patients treated by radiotherapy alone. Approximately 80% of patients completed chemotherapy according to the protocol guidelines.
In China, Shi[1] modified the concomitant boost schedule designed by Anderson Cancer Center to LCAF radiotherapy on SCC of the esophagus. The results were very encouraging. The 5-year survival and local control rate were markedly improved in the LCAF group. Compared with CF radiotherapy, the 5-year overall survival was 34% versus 15% which was statistically significant, the local control rate was 55% versus 21%. Henceforth, more randomized and retrospective trials confirmed the results[7-9]. LCAF radiotherapy is the most frequently used radiotherapeutic management for localized SCC of esophageal carcinoma in some cancer hospitals in China including our department.
The survival rate and local control rate are comparable with operation and chemoradiotherapy in the patients treated with LCAF radiotherapy alone. Esophagus can be preserved, and the severe complications can be decreased. In the retrospective study, most patients were clinical stage T2N0M0, at upper thoracic and middle thoracic. The 5-year survival rate was 47.8%, and the 5-year local control rate was 84.5%. Grade 3-5 acute radiation-induced toxicities were 16.1%. The incidence of grade 3-5 late complications was 7.0%.
In analyzing failure patterns, there was significant difference between the patients in early-stage and advanced-stage. Local/regional recurrence was the main reason of failure in the advanced patients treated with conventional radiotherapy. Forty-two percent patients died of local recurrence, and nineteen percent patients died of metastasis in the advanced patients treated with LCAF radiotherapy[1]. Forty-five percent patients had local regional disease as the first failure, distant metastasis as the first failure occurred in 13% of concurrent chemoradiotherapy[2]. In our retrospective study, distant metastasis rate (28.6%) increased significantly, and local regional recurrent rate (12.5%) decreased markedly.
Edited by Wen CY
| 1. | Shi XH, Yao W, Liu T. Late course accelerated fractionation in radiotherapy of esophageal carcinoma. Radiother Oncol. 1999;51:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Murakami M, Kuroda Y, Nakajima T, Okamoto Y, Mizowaki T, Kusumi F, Hajiro K, Nishimura S, Matsusue S, Takeda H. Comparison between chemoradiation protocol intended for organ preservation and conventional surgery for clinical T1-T2 esophageal carcinoma. Int J Radiat Oncol Biol Phys. 1999;45:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230:392-400; discussion 400-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 404] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Vigneswaran WT, Trastek VF, Pairolero PC, Deschamps C, Daly RC, Allen MS. Extended esophagectomy in the management of carcinoma of the upper thoracic esophagus. J Thorac Cardiovasc Surg. 1994;107:901-96; discussion 901-96;. [PubMed] |
| 5. | Zenone T, Romestaing P, Lambert R, Gerard JP. Curative non-surgical combined treatment of squamous cell carcinoma of the oesophagus. Eur J Cancer. 1992;28A:1380-1386. [PubMed] |
| 6. | Roca E, Pennella E, Sardi M, Carraro S, Barugel M, Milano C, Fiorini A, Giglio R, Gonzalez G, Kneitschel R. Combined intensive chemoradiotherapy for organ preservation in patients with resectable and non-resectable oesophageal cancer. Eur J Cancer. 1996;32A:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Wang WD. Conventional radiotherapy boosted with late course hyperfractionation in patient with esophageal cancer (abstr.). Zhonghua Fangshe Zhongliuxue Zazhi. 2001;10:30. |
| 8. | Wang Y, Shi XH, He SQ, Yao WQ, Wang Y, Guo XM, Wu GD, Zhu LX, Liu TF. Comparison between continuous accelerated hyperfractionated and late-course accelerated hyperfractionated radiotherapy for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Zhao KL, Wang Y, Shi XH. Analysis of outcome and failure reasons of late accelerated hyperfrationation radiotherapy for esophageal carcinoma. Zhonghua Fangshe Zhongliuxue Zazhi. 2000;10:14-16. |