INTRODUCTION
Tumor immunity is primarily cell-mediated. The tumor antigen leads to anti-tumor immune response of the host. A body of evidences have shown that the specific activation of lymphocytes requires two signals; one is provided by T-cell receptor complex coupled to CD3/MHC peptide antigen, and the other is costimulatory signal delivered by interaction of costimulatory molecules with their ligands expressed on antigen presenting cells (APCs). In the absence of costimulatory signals, antigen-MHC complex interaction may lead to T-cell clonal anergy or deletion, and thus, the effective cellular immune response can not be induced. Furthermore, there are evidences that activation of lymphocyte subpopulation with different function needs unique costimulation factors. Therefore, investigation of costimulators on infiltrating lymphocytes and tumor cells at the tumor site and of their significance will be of great help in developing new methods of anti-tumor immunotherapy. Cell-mediated immunity is the main mechanism of anti-tumor immunity of the host. With presentation of antigen recognization and deepening of the understanding of activated T-cells, it is believed that in the presence of costimulatory signals, effective anti-tumor immune response presents tumor antigen multipeptide to T cells and stimulates T-cell immune response. B7 is an important costimulatory molecule. Most tumors lack or have a low expression of B7-1, so they do not induce effective anti-tumor immune response. The tumor cells escape from immune system of the host and continue to grow[1-15]. Although many in vitroexperiments support this idea, in situ studies on human tumors have shown that most tumor tissues express the B7-1 or B7-2 molecules. So the idea meets challenges. An in vitro study on new members of B7 family - B7H1, B7H2 and ICOS suggested that the role of ICOS-B7H costimulatory pathway stimulated IL-10 production and induced secretion of Th2 type cytokines. The present study was to explore the role and biological significance of ICOS-B7H costimulatory signals and the possible mechanisms in tumor escape, and to provide the theoretical foundation for designing effective schemes of immunotherapy of carcinoma.
MATERIALS AND METHODS
Patients
17 patients with gastric carcinoma (10 matastatic, and 7 non-matastatic) and 6 patients with gastric ulcer were identified by pathological diagnosis at the Department of Pathology, Second Hospital of Xi'an Jiaotong University. The fresh tissue samples were instantly fixed in 10% formalin, and embeded in paraffin according to routine procedures. Tonsil tissue served as a positive control.
Main reagents
Digoxigenin (DIG)-labeling and detection kits were purchased from Boehringer Mannheim Company in Germany. Solution for in situ hybridization was mixed with diethyl pyrocarbonate (DEPC) water. All apparatuses were baked at 180 °C for four hours and DEPC water was digested with RNase.
Specimens
Sections (4-5 μm) cut from the tissue block were de-paraffined in 65 °C oven for 24 h and then stored at 70 °C until being used.
Labeling of oligonucleotide probes and detection of their sensitivities
Labeling On the ice, 4 μL reaction buffer (vial 1), 4 μL CoCl2 solution (vial 2), 1 μL probe (100 pmol), 1 μL DIG-dUTP solution (vial 2), 1 μL dATP solution (vial 4), 1 μL terminal transferase (vial 5) and 8 μL DEPC water, were consecutively added into an eppendorf tube, and carefully mixed and incubated at 37 °C for 15 min. 2 μL stopping solution (prepared by mixing 1 μL glycogen solution (vial 9) with 200 μL 0.2 mol/L EDTA solution) was added into the tube to stop the labeling reaction. Then, 2.5 mL 4 mol/L CrCl2 and 75 μL pro-cold pure alcohol were added to deposit the labeled. After the supernatants were drained out, the probe was washed with 50 μL 70% pre-cold alcohol and dried with a frozen dry machine, and stored at 20 °C until being used.
Detection of sensitivity Sensitivity of each probe was measured by the detection kit following the manufacturer'sinstructions. The lowest concentration for positive staining was 1.25 pmol/ml. The oligonucleotide probe sequences were as follow. B7-1 5'-CATGAAGCTGTGGTTGGTTG-3'; B7H1 5'-TGCTTGTCCAGGTGACTTCG-3'; B7H2 5'-CCATCGCTCTGACTTCCTTC-3'; and ICOS 5'-TTCAGCTGGCAACAAAGTTG-3'.
Hybridization
Sections stored at 70 °C were recovered at room temperature, deparaffined in dimethylbenzene, and hydrated in gradient alcohol. They were digested in 0.5 μg/mL fresh proteinase K solution at room temperature for 20 min, and fixed in 4% poly-formaldehyde for 20 min after digestion was stopped in 0.2% glycine, and then repeatedly rinsed with 1 × PBS for 10 min. The sections were treated in 0.2N HCl solution at room temperature for 10 min, rinsed with DEPC water for 3 min; and dehydrated in gradient alcohol. At 42 °C in a bio-hybridization oven, all sections were pre-hybridized for 2 h, and then hybridized for 20 h. After hybridization, they were rinsed with 2 × SSC for two times, 15 min each, rinsed with 1 × SSC for 30 min and finally with buffer I solution (pH7.5) for 6 min.
Detection
50 μL blocking reagent (containing 8% normal goat serum, 0.3% Triton X-100 buffer I) was instilled onto each section. The sections were incubated at 37 °C for one hour and a half, and then 30 mL anti-DIG-AP conjugate (1:500) was added onto each section, and all sections were incubated at 37 °C for two hours and rinsed with buffer I two times, 15 min each, then rinsed with buffer III (100 mmol/L Tris-HCl, 100 mmol/L NaCl and 50 mmol/L MgCl2, pH9.5) for 5 min, and stained with NBT-BCIP at 20 °C for 12 h. Finally, the reaction was stopped with buffer IV solution (10 mmol/L Tris-HCl, and 1 mmol/L EDTA, pH8.0), and the sections were washed in running water, counterstained with methyl green, dehydrated, cleaned in xylene and coverslipped.
Evaluation of results
Under a light microscope, positively hybridized signals were mainly located in the infiltrating lymphocytes and colored purple blue. Reactivity was scored using a semi-quantitative method. First, each section was randomly scored one thousand cells. The positive cell ratio in each section was calculated. Then, according to the staining intensity of positive cells, results were graded as following: intense 3+, moderate 2+, weak 1+ and negative, a score of 3, 2, 1, 0 was assigned respectively. Finally, the positive cell ratio multiplied with staining intensity score of the positive cells was regarded as positive index of mRNA expression of costimulatory molecules.
Control experiment
Expression of ICOS mRNA of the chronic tonsillitis lymphocytes was regarded as a positive control. The results were negative control when no probe was used.
Statistical anslysis
All data were expressed as ¯x ± s and analyzed by SPSS statistic software. Differences in values were considered significant if P < 0.05.
RESULTS
mRNA expression of costimulatory molecules within tissues of human gastric carcinoma
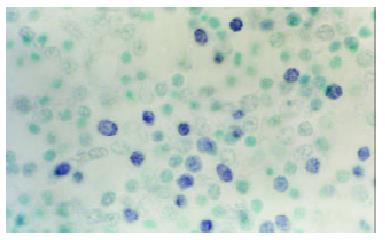
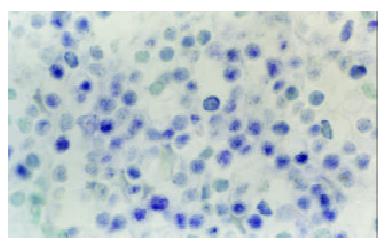
At the site of gastric carcinoma, positive cells for B7H1, B7H2, ICOS were mainly tumor infiltrating lymphocytes (Figure 1). Some positive cells were tumor cells. Their mRNA positive expression indexes were 0.512 ± 0.333, 0.812 ± 0.454 and 0.702 ± 0.359, respectively. B7-1 mRNA was expressed on tumor cells and TILs (Figure 2), with a positive expression index being 0.293 ± 0.253. The mRNA expression levels of B7H1, B7H2 and ICOS were significantly higher than that of B7-1(all P < 0.05).
Figure 1 B7H1 mRNA expression of tumor infiltrating lymphocytes (TILs) within tissues of human gastric carcinoma (In situ hybridization, NBT-BCIP Staining, counterstained with methyl green, × 1000).
Figure 2 B7-1 mRNA expression of tumor infiltrating lymphocytes (TILs) within tissues of human gastric carcinoma (In situ hybridization, NBT-BCIP Staining, counterstained with methyl green, × 1000)
Expression of costimulatory molecules in relation to metastasis of gastric carcinoma
The expression levels of B7-1, B7H1, B7H2 and ICOS had no statistical significance between metastasis and non-metastasis of gastric carcinoma (P > 0.05), which suggested that expressions of costimulatory molecules at tumor site were not correlated to the metastasis of tumor.
Expression of costimulatory molecules in relation to differentiation of gastric carcinoma
mRNA expression levels of B7-1, B7H1 and ICOS on infiltrating lymphocytes at the tumor site was not significantly different between well-differentiated and poorly-differentiated. However, the expression level of B7H2 mRNA was significantly higher in well-differentiated than in poorly-differentiated (1.040 ± 0.400 vs 0.651 ± 0.436, P = 0.047). mRNA expression of B7-1 was greatly lower than that of B7H1, B7H2 and ICOS in both well-differentiated and poorly-differentiated gastric carcinoma which might indicate that function of B7-1 was different from other costimulatory molecules in gastric carcinoma.
Expression of costimulatory molecules in comparison between gastric carcinoma and gastric ulcer
In the tissue of gastric ulcer, the expression levels of B7-1, B7H1, B7H2 and ICOS on infiltrating lymphocytes were higher than those in gastric carcinoma. But there was no difference in the expression of B7H1, B7H2 and ICOS between the tissues of gastric carcinoma and gastric ulcer except B7-1. The effect of costimulators in the gastric ulcer was not clear.
DISCUSSION
Cell-mediated immunity is the main mechanism of antitumor immunity of the host. With presentation of antigen identification and deepening of the understanding of T-cells activated, it is believed that in the presence of costimulatory signals, effective antitumor immune response presents tumor antigen multipeptide to T cells and stimulates T-cell immune response[16-18]. B7 is an important costimulatory molecule. Most tumors lack or have a low expression of B7-1, so they do not induce effective antitumor immune response. The tumor cells escape from immune system of the host and continue to grow. Though many in vitro experiments support this idea, in situ studies on human tumors have shown that most tumor tissues express the B7-1 or B7-2 molecules[19]. So the idea meets challenges.
The effective anti-tumor immune response requires three factors, including immunogenicity, costimulatory signals and Th1 type cytokines. In the past, it was emphasized that cytokines or/antigen essence determined the classification of immune responses. Generally, weak immunogenicity of tumor cells primarily induced Th2 response[20]. In recent years, studies on costimulatory signals in vitro have shown that different costimulatory signals play a determinant role in immune response type. There might be Th2 predominant costimulatory signals in human tumor site. The present study has detected mRNA expression of B7H1, B7H2 and ICOS within the tissue of human gastric carcinoma, using in situ hybridization with DIG-labeled oligonucleotide probes. The results suggest that tumor infiltrating lymphocytes in tissue of gastric carcinoma express high levels of recently discovered members of B7 costimulatory family, B7H1, B7H2 and ICOS[21-26]. Compared with mRNA expression level of B7-1, difference was dramatically significant. But mRNA expression levels of B7H1, B7H2 and ICOS were not correlated to the pathological grade and metastasis of gastric carcinoma. The experiments[27-35] in vitro have verified that the interaction between B7h and ICOS stimulates the proliferation of T-cells and production of Th2 cytokines, preferentially secretion of IL-10. This is probably the main reason that Th2 cytokines may predominate at the tumor immune- microenvironment of gastric carcinoma, and thus the type of immune response shifts from Th1 to Th2. There was an increase of Th2 cytokine-IL-10 in tumor immune-microenvironment of gastric carcinoma[36].
In this study, we have also observed the expression of B7-1 in the tissue of gastric carcinoma, and found that tumor infiltrating lymphocytes (TIL) and tumor cells expressed low gastric carcinoma levels of B7-1 mRNA. Now that there were costimulatory molecules in the tissues of gastric carcinoma, why did not the host produce the effective antitumor immune response With the further study of immunology and tumor immunology, a new concept related to interaction between TIL and tumor cells was introduced. That is the tumor microenvironment. This theory supposes that immune system of the host cannot eliminate tumors because there are many inhibitors of tumor immunity at the site of tumor microenvironment. For example, tumor cells produce TGF-β, PGE2 and so on, which are accumulated at tumor site and interfere with the activity of recruited immunocytes. Activated immunosuppressive cells further augment the immuno-depression mechanism in tumor microenvironment. In addition, a recent study[27] has shown that B7-1 costimulation also enhances the up-regulation effects of ICOS costimulation which results in the increase of Th2 cytokines, preferentially inducing the secretion of IL-10.
The present experimental results suggest that the tumor infiltrating lymphocytes in the tissues of gastric carcinoma express high levels of B7H1, B7H2 and ICOS mRNA. Some tumor cells also express them but the expression levels are low. This indicates that there are costimulatory signal pathways in the site of gastric carcinoma. Since the interaction between B7H1, B7H2 and ICOS stimulates proliferation of T cells by various ways and induces secretion of IL-10. IL-10 is known to be an immune inhibitor, which down-regulates function of Th1 type cells, and inhibits antitumor immune response of the body, and thus the body is in the state of immune inhibition. Therefore, it is supposed that although interaction between B7H1, B7H2 and ICOS delivers costimulatory signals to T-cells, promotes their activation, the effective antitumor immunity can not be induced. Tumor cells can escape from the immunosurveillance of the host and continue to grow. This is due to the fact that many inhibitors at tumor site have blocked the role of specifically activated T cells. As far as most tumors are concerned, the host’s immunoreaction can not eliminate the tumor because Th2 type response is stimulated, or it can be recognized by immune system of the body. Maybe there are other regulation mechanisms. The present study indicates that costimulatory pathways might exist at the tumor site with different functions. ICOS-B7h pathway is predominant and plays an important role in negative regulation of cell-mediated immune response. This finding might provide a new approach for designing effective immune-therapy of carcinoma.