Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1365
Revised: March 10, 2003
Accepted: March 16, 2003
Published online: June 15, 2003
AIM: To estimate the age-specific prevalence of anti-ulcer drug use and to calculate the usage of different anti-ulcer drugs over 5 years within the universal health insurance program in Taiwan area.
METHODS: The National Health Insurance Research Database in Taipei supplied the cohort data sets of 200000 people. The ambulatory and inpatient claims of the cohort from 1997 to 2001 were analyzed. The anti-ulcer drugs included all drug items of the group A02B (drugs for treatment of peptic ulcer) in the Anatomical Therapeutic Chemical classification system (version 2000). The amount of drug usage was measured in unit of defined daily dose.
RESULTS: Among the totally 13034393 visits with 56672631 ambulatory prescription items, there were 398150 (0.7%) prescribed items of anti-ulcer drugs in 378855 (2.9%) visits. Among the 107649 admissions with 5762312 inpatient prescription items, there were 24598 (0.4%) prescribed items of anti-ulcer drugs in 11548 (10.7%) admissions. The annual prevalence of anti-ulcer drug use was 9.6% in 1997, 11.6% in 1998, 15.4% in 1999, 14.5% in 2000, and 15.9% in 2001 respectively. The 5-year prevalence was 36.1%. The age-specific prevalence among the people younger than 20 years was 9.2% in 2001 and 23.7% during the 5-year period. Cimetidine not only was the most popular ingredient among anti-ulcer drugs (57634 cimetidine users in 70729 all anti-ulcer drug users during the 5-year period) but also had the largest prescribed amount (42.3% of DDDs for all anti-ulcer drug users during the 5-year period). The annually prescribed amount of anti-ulcer drugs had grown from 4.9 DDDs/1000 inhabitants/day in 1997 to 7.5 in 2001. This increase was largely attributed to H2-receptor antagonists and the expanding number of users.
CONCLUSION: Prescribing of anti-ulcer drugs is indeed popular among the Chinese population in Taiwan area. The disproportionate use of anti-ulcer drugs by children demands further investigation.
- Citation: Chen TJ, Chou LF, Hwang SJ. Prevalence of anti-ulcer drug use in a Chinese cohort. World J Gastroenterol 2003; 9(6): 1365-1369
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1365.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1365
In the past three decades, the invention of several revolutionary anti-ulcer drugs, e.g. H2-receptor antagonists, synthetic prostaglandins, proton pump inhibitors, and cytoprotective agents, has changed the physicians' treatment patterns in gastroenterology and greatly improved the ulcer-healing rate of patients with peptic ulcer disease[1-6]. In spite of effectiveness and popularity, the cost of these drugs has also aroused concern in the health care systems of developed countries[7-11]. The concern has been aggravating in recent years because of expanding use of proton pump inhibitors in treating gastroesophageal reflux disease. Although prescribing of these potent acid-suppressing drugs is popular, their patterns of utilization have been infrequently documented in national surveys[12-21].
In Taiwan area, a single and universal health insurance program started in 1995 and covered nearly all inhabitants (21653555 beneficiaries at the end of 2001)[22]. The prescription drug benefits are included in the insurance. Because all claims data for the reimbursement purpose are in electronic form and available to researchers, we can perform a survey of anti-ulcer drug use among the Chinese population in Taiwan area.
The aims of this study were twofold: to estimate the age-specific prevalence of anti-ulcer drug use and to calculate the usage of different anti-ulcer drugs over 5 years within the universal health insurance program in Taiwan area. The strengths of our study were to use the longitudinal data sets of a representative cohort of 200000 people and to adopt the unit of international standards in measuring the anti-ulcer drug usage.
We obtained 4 cohort data sets (R01-4) from the National Health Insurance Research Database (NHIRD; http://www.nhri.org.tw/nhird/) in Taipei in November 2002. The total 200000 people in these 4 cohort data sets had been randomly sampled from 23753407 people who were ever insured under the universal health insurance program in Taiwan area from March 1, 1995 to December 31, 2000. Not every person of the cohort was insured through the study period because of new birth, death, immigration, and emigration. The cohort data sets contained all insurance claims of 200000 people from 1996 to 2001. The structure of the insurance claim files had been described in details in our previous study[23].
In the current study, we analyzed the ambulatory and inpatient files of the cohort data sets from 1997 to 2001. Totally, there were 13034393 visits, 56672631 ambulatory prescription items, 107649 admissions, and 5762312 inpatient prescription items.
Besides, we obtained a complete file of 21146 approved drug items of Western medicine in Taiwan area from the web site of the Bureau of National Health Insurance (BNHI; http://www.nhi.gov.tw/; accessed January 12, 2002). Each drug of different brand, strength and form was officially assigned a unique code for use in the claims file. The BNHI also offered a list of ATC codes (the Anatomical Therapeutic Chemical classification system, version 2000)[24] for each drug item.
The anti-ulcer drugs in our study included all drug items of the group A02B (drugs for treatment of peptic ulcer, renamed to 'drugs for peptic ulcer and gastroesophageal reflux disease' in 2002) in the ATC classification system. This group of drugs has 5 subgroups of the fourth level: A02BA (H2-receptor antagonists, H2RA), A02BB (prostaglandins), A02BC (proton pump inhibitors, PPI), A02BD (combinations for eradication of Helicobacter pylori), and A02BX (other drugs for treatment of peptic ulcer, renamed to 'other drugs for peptic ulcer and gastroesophageal reflux disease' in 2002). A total of 428 anti-ulcer drug items, including the original brands and generics, have been registered in Taiwan area since 1995. Some drugs might be no more available on the market or not reimbursable by the insurance during the study period.
In estimating the age-specific prevalence of anti-ulcer drug use, we first identified the people receiving anti-ulcer drug items in each year. Because the number of people in the cohort fluctuated during the study period, we calculated the number of the denominator in each year by excluding those people who were not insured at any time of that year. A person's age in a year was defined as the difference between her/his birthday and the end of that year. In estimating the 5-year age-specific prevalence from 1997 to 2001, we took December 31, 2001 as the index date to calculate a person's age.
In describing the distribution of anti-ulcer drug prescriptions among the cohort, we calculated the number of recipients and the total prescribed amount for each ingredient (ATC 5th level) in each year. Supposed that the cohort did not take anti-ulcer drugs before the base year of 1997, the number of new anti-ulcer drug users was additionally computed for each year after 1997. The prescribed amounts of anti-ulcer drugs were measured in unit of defined daily dose (DDD) by ATC classification system[24]. The original dose of each prescription was converted to a number of DDDs according to the DDD of the ingredient. Some anti-ulcer drugs (e.g. cetraxate, urogastrone, and gefarnate) lacked either ATC codes or DDDs; we used the most commonly prescribed daily doses as their DDDs. For international comparison, the numbers of DDDs per 1000 inhabitants per day were also computed.
The database software of Microsoft SQL Server 2000 was used for data linkage and processing. The regular statistics were displayed.
Among the 200000-people cohort, only 195971 people were eligible during the 5-year study period. The other 4029 people who had dropped out of the insurance before 1997 would not be included in the following analyses. The number of eligible people varied from year to year (Table 1). There were more men than women (100257 vs. 95654), and the status of sex was unknown in 60 persons.
| 1997 | 1998 | 1999 | 2000 | 2001 | 1997-2001 | |
| Sampling cohort | ||||||
| 0 – 19 years | 57388 | 56427 | 55448 | 54684 | 51029 | 52103 |
| 20 – 39 years | 62613 | 63765 | 64848 | 65219 | 63800 | 69425 |
| 40 – 59 years | 39809 | 41420 | 42922 | 44241 | 45432 | 47663 |
| 60 years and older | 20971 | 21836 | 22489 | 23093 | 23715 | 26780 |
| Total | 180781 | 183448 | 185707 | 187237 | 183976 | 195971 |
| Patients with anti-ulcer drugs | ||||||
| 0 – 19 years | 2965 | 3611 | 5171 | 4634 | 4712 | 12339 |
| 20 – 39 years | 5345 | 6741 | 9249 | 8788 | 9673 | 23741 |
| 40 – 59 years | 4933 | 6213 | 8277 | 8113 | 8664 | 20166 |
| 60 years and older | 4172 | 4787 | 5905 | 5643 | 6132 | 14483 |
| Total | 17415 | 21352 | 28602 | 27178 | 29181 | 70729 |
| Prevalence of anti-ulcer drug use | ||||||
| 0 – 19 years | 5.2% | 6.4% | 9.3% | 8.5% | 9.2% | 23.7% |
| 20 – 39 years | 8.5% | 10.6% | 14.3% | 13.5% | 15.2% | 34.2% |
| 40 – 59 years | 12.4% | 15.0% | 19.3% | 18.3% | 19.1% | 42.3% |
| 60 years and older | 19.9% | 21.9% | 26.3% | 24.4% | 25.9% | 54.1% |
| Total | 9.6% | 11.6% | 15.4% | 14.5% | 15.9% | 36.1% |
During the 5-year study period, 356 distinct anti-ulcer drugs had existed in the cohort data sets. The drugs belonged to 18 ingredients of ATC 5th level. At the ambulatory sector, there were 398150 (0.7%) prescribed items of anti-ulcer drugs in 378855 (2.9%) visits; at the inpatient sector, there were 24598 (0.4%) prescribed items in 11548 (10.7%) admissions.
In 1997, as high as 9.6% (17414/180781) of eligible cohort received anti-ulcer drugs. The percentage increased by two-thirds to 15.9% (29181/183976) in 2001. More than a third (36.1%) of the cohort had ever received anti-ulcer drugs during the 5-year study period. Generally, the prevalence of anti-ulcer drug use increased with age. Another noteworthy finding was that anti-ulcer drugs had been prescribed to an appreciable percentage of children and adolescents (Table 1).
Cimetidine was the most popular ingredient of anti-ulcer drugs among the cohort, followed by sucralfate, ranitidine, famotidine, omeprazole, pirenzepine, and lansoprazole (Table 2). The majority of new anti-ulcer drug users in each year were also attributed to cimetidine.
| ATCa coding | Group/ingredient name | 1997 | 1998 | 1999 | 2000 | 2001 | 1997-2001 |
| A02BA H2 RA | |||||||
| 01 Cimetidine | 11538 | 15793 (11340) | 22905 (14525) | 22356(10564) | 24305 (9667) | 57634 | |
| 02 Ranitidine | 1588 | 1852 (1482) | 2262 (1757) | 2283 (1611) | 2579 (1802) | 8240 | |
| 03 Famotidine | 974 | 1143 (953) | 1401 (1113) | 1526 (1216) | 1669 (1232) | 5488 | |
| 04 Nizatidine | 187 | 255 (221) | 295 (236) | 199 (157) | 137 (108) | 909 | |
| 06 Roxatidine | 89 | 98 (82) | 87 (76) | 83 (67) | 97 (80) | 394 | |
| A02BB Prostaglandins | |||||||
| 01 Misoprostol | 128 | 145 (129) | 156 (135) | 150 (112) | 88 (68) | 572 | |
| A02BC PPIS | |||||||
| 01 Omeprazole | 709 | 913 (807) | 1168 (990) | 1150 (917) | 1445 (1156) | 4579 | |
| 02 Pantoprazole | - | 1(1) | 84 (84) | 147 (133) | 273 (255) | 473 | |
| 03 Lansoprazole | 151 | 391 (365) | 531 (459) | 647 (537) | 785 (643) | 2155 | |
| 04 Rabeprazole | - | - | - | - | 31 (31) | 31 | |
| A02BX Other drugs | |||||||
| 01 Carbenoxolone | 287 | 260 (219) | 337 (271) | 155 (105) | 199 (164) | 1046 | |
| 02 Sucralfate | 3463 | 3192 (2510) | 3107 (2301) | 2390 (1506) | 2173 (1390) | 11170 | |
| 03 Pirenzepine | 1177 | 1197 (937) | 1181 (857) | 731 (454) | 625 (351) | 3776 | |
| 05 Bismuth subcitrate | 329 | 299 (266) | 271 (223) | 173 (141) | 142 (111) | 1070 | |
| 06 Proglumide | 113 | 61 (53) | 36 (34) | 3 (3) | 8 (5) | 208 | |
| 07 Gefarnate | 248 | 295 (270) | 416 (363) | 326 (265) | 203 (161) | 130 7 | |
| - Cetraxate | 356 | 101 (81) | 72 (59) | 48 (40) | 41 (37) | 57 3 | |
| - Urogastrone | 192 | 46 (34) | 42 (32) | 14 (12) | 6 (5) | 275 | |
| Total | 17415 | 21352 (14423) | 28602(16611) | 27178(11660) | 2918123(10622) | 70729 | |
Measured in unit of DDDs, cimetidine again had the largest prescribed amount (42.3%) of all anti-ulcer drugs among the cohort during the 5-year study period (Table 3). It was then followed by ranitidine (16.2%), famotidine (10.8%), omeprazole (9.7%), and lansoprazole (4.5%). The majority of anti-ulcer drugs were used at the ambulatory sector (93.7% of total DDDs).
| ATCa coding | Group/ingredient name | 1997 | 1998 | 1999 | 2000 | 2001 |
| A02BA H2 RA | ||||||
| 01 Cimetidine | 114145 | 151403 | 221528 | 198255 | 221836 | |
| 02 Ranitidine | 57329 | 62357 | 69780 | 69954 | 88034 | |
| 03 Famotidine | 33366 | 40063 | 51198 | 52852 | 53108 | |
| 04 Nizatidine | 8943 | 12304 | 12282 | 8269 | 5305 | |
| 06 Roxatidine | 3493 | 3057 | 3215 | 2847 | 4460 | |
| A02BB Prostaglandins | ||||||
| 01 Misoprostol | 2244 | 3649 | 5594 | 4534 | 1941 | |
| A02BC PPIS | ||||||
| 01 Omeprazole | 26635 | 33587 | 46429 | 43589 | 57193 | |
| 02 Pantoprazole | - | 14 | 2593 | 4845 | 9276 | |
| 03 Lansoprazole | 5840 | 14276 | 19624 | 24860 | 31023 | |
| 04 Rabeprazole | - | - | - | - | 864 | |
| A02BX Other drugs | ||||||
| 01 Carbenoxolone | 5708 | 4164 | 4768 | 1945 | 1918 | |
| 02 Sucralfate | 26172 | 20170 | 15942 | 10960 | 9898 | |
| 03 Pirenzepine | 7575 | 7378 | 5925 | 3868 | 3942 | |
| 05 Bismuth subcitrate | 7999 | 6104 | 5213 | 2686 | 2754 | |
| 06 Proglumide | 1010 | 459 | 198 | 12 | 31 | |
| 07 Gefarnate | 14379 | 17859 | 26568 | 16682 | 8972 | |
| - Cetraxate | 4072 | 1254 | 849 | 615 | 657 | |
| - Urogastrone | 5067 | 1272 | 1151 | 232 | 32 | |
| Total | 323976 | 379370 | 492856 | 447005 | 501243 | |
| Ambulatory sector | 297213 | 355837 | 465326 | 420144 | 470924 | |
| Inpatient sector | 26764 | 23532 | 27530 | 26861 | 30319 | |
| DDDs/1000 inhabitants/day | 4.9 | 5.7 | 7.3 | 6.5 | 7.5 | |
The total prescribed amount of anti-ulcer drugs grew from 4.9 DDDs/1000 inhabitants/day in 1997 to 7.5 in 2001 (Table 3). This increase was attributed to the expanded number of users because the average prescribed amount of anti-ulcer drugs per user in a year remained relatively stable (18.7 ± 54.8 DDDs in 1997, 17.9 ± 41.0 in 1998, 17.3 ± 51.0 in 1999, 16.5 ± 40.5 in 2000, and 17.3 ± 40.8 in 2001).
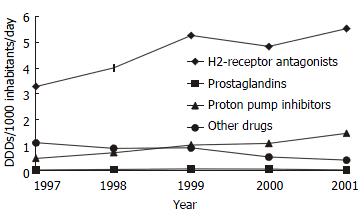
On the other hand, H2-receptor antagonists and proton pump inhibitors had contributed to the growth of the total prescribed amount of anti-ulcer drugs during the study period (Figure 1). While H2-receptor antagonists had the largest share of growth, proton pump inhibitors had the highest growth rate. In the meantime, the usage of prostaglandins had remained stable, but other drugs for treatment of peptic ulcer had fewer users and smaller prescribed amount totally.
To the best of our knowledge, our study might be one of the few reports that surveyed the anti-ulcer drug use in the Chinese population. Only with the computerization of insurance reimbursement, pharmacoepidemiological studies of such a large scale could be feasible. Besides, the person-based sampling in our study could estimate both the total amount and prevalence of drug use among the population.
Our study revealed that prescribing of anti-ulcer drugs was indeed popular in Taiwan. Nearly a sixth of the population received anti-ulcer drugs covered by the health insurance in 2001 and more than a third of the population had been exposed to such drugs during the 5 years. But the total usage of anti-ulcer drugs in Taiwan was not high in international comparison. According to the statistics of the OECD (Organization for Economic Co-operation and Development), 8 countries supplied their national consumption of anti-ulcer drugs in 1998: Australia (38.8 DDDs/1000 inhabitants/day), Sweden (29.0), Iceland (28.1), Denmark (16.5), Norway (16.0), Czech Republic (13.1), Finland (12.4), and Slovakia (8.7)[25]. In contrast, Taiwan had only 5.7 DDDs/1000 inhabitants/day of anti-ulcer drugs in the same year. However, the statistics has not been adjusted by age.
In the 1990s, the developed countries experienced a drastic increase of anti-ulcer drug consumption since the introduction of proton pump inhibitors. For example, the national consumption of anti-ulcer drugs in Sweden increased from 9.2 DDDs/1000 inhabitants/day in 1990 to 34.4 in 2000[25]. During the 5 years of our study, a growing trend of anti-ulcer drugs was also observed in Taiwan. But the increase was largely attributed to H2-receptor antagonists and expanding user group. The explanation might be that the reimbursement policy of the health insurance in Taiwan limited the use of expensive proton pump inhibitors on the one hand and loosened the regulation over the much cheaper generics of H2-receptor antagonists on the other hand.
While overuse of proton pump inhibitors has become a research topic[26,27], our study found that at least the children in Taiwan might be disproportionately exposed to anti-ulcer drugs. Because children were generally not able to receive upper gastrointestinal endoscopy, their use of anti-ulcer drugs could be seldom justified. It demanded further studies to explore such a situation in Taiwan.
Our study with insurance claims in Taiwan had some limitations. At first, the drug use outside the insurance was not included in the analysis. However, the majority of anti-ulcer drugs, including the low-dose cimetidine, were prescription-only drugs in Taiwan. Besides, the compulsory health insurance covered nearly all inhabitants in Taiwan and reimbursed most prescription-only drugs. The use of anti-ulcer drugs at the private market should be of a less significant scale.
Secondly, the actual duration of drug treatment was not computed in our study because of missing dosage frequency in the inpatient files of the NHIRD data sets. Instead, we calculated the cumulated numbers of DDDs for each person as a proxy of treatment duration. But the DDD is arbitrarily set for trend and international comparisons. It does not consider the dosing at the specific conditions of children, elderly, and other risk groups. However, our data showed that the yearly amount of anti-ulcer drugs per user was low on average. It might be inferred that most people took anti-ulcer drugs only for a short term.
Thirdly, the purpose of cohort data sets in the NHIRD was to trace a cohort retrospectively and prospectively. The people of the cohort were chosen in 2000 and it was planned to follow them up continuously in the next years. Thus, the data sets of 2001 did not include anyone born after December 31, 2000. The denominator in 2001 should be smaller than the actual number of people and the prevalence correspondingly became a little overestimated.
Finally, we did not analyze the distribution of diagnoses in our study because a claims diagnosis served for the purpose of reimbursement and was seldom verified. The NHIRD data sets did provide the information whether the patients had received the endoscopic or radiological examinations of upper gastrointestinal tract. But no laboratory findings were routinely transmitted to the insurer in electronic form. Conventional epidemiological surveys are still needed to understand the prevalence of peptic ulcer and gastroesophageal reflux disease in Taiwan.
This study was based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes in Taiwan. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
Edited by Xu XQ
| 1. | Molinder H, Wallander MA, Svärdsudd K, Bodemar G. The introduction of H2-receptor antagonists to Scandinavia: effects of experts' opinions. Scand J Gastroenterol. 1998;33:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Soll AH. Consensus conference. Medical treatment of peptic ulcer disease. Practice guidelines. Practice Parameters Committee of the American College of Gastroenterology. JAMA. 1996;275:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 117] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 220] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:1434-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 252] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Andersen IB, Bonnevie O, Jørgensen T, Sørensen TI. Time trends for peptic ulcer disease in Denmark, 1981-1993. Analysis of hospitalization register and mortality data. Scand J Gastroenterol. 1998;33:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Thors H, Svanes C, Thjodleifsson B. Trends in peptic ulcer morbidity and mortality in Iceland. J Clin Epidemiol. 2002;55:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | McGavock H, Webb CH, Johnston GD, Milligan E. Market penetration of new drugs in one United Kingdom region: implications for general practitioners and administrators. BMJ. 1993;307:1118-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | McManus P, Marley J, Birkett DJ, Lindner J. Compliance with restrictions on the subsidized use of proton pump inhibitors in Australia. Br J Clin Pharmacol. 1998;46:409-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Cromwell DM, Bass EB, Steinberg EP, Yasui Y, Ravich WJ, Hendrix TR, McLeod SF, Moore RD. Can restrictions on reimbursement for anti-ulcer drugs decrease Medicaid pharmacy costs without increasing hospitalizations. Health Serv Res. 1999;33:1593-1610. [PubMed] |
| 10. | O'Connor JB, Provenzale D, Brazer S. Economic considerations in the treatment of gastroesophageal reflux disease: a review. Am J Gastroenterol. 2000;95:3356-3364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Lucas LM, Gerrity MS, Anderson T. A practice-based approach for converting from proton pump inhibitors to less costly therapy. Eff Clin Pract. 2001;4:263-270. [PubMed] |
| 12. | Thors H, Sigurdsson H, Oddsson E, Thjodleifsson B. Survey of prescriptions for peptic ulcer drugs (ACT class AOB2) in Iceland. Scand J Gastroenterol. 1994;29:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Roberts SJ, Bateman DN. Prescribing of antacids and ulcer-healing drugs in primary care in the north of England. Aliment Pharmacol Ther. 1995;9:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Goudie BM, McKenzie PE, Cipriano J, Griffin EM, Murray FE. Repeat prescribing of ulcer healing drugs in general practice--prevalence and underlying diagnosis. Aliment Pharmacol Ther. 1996;10:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Moride Y, Melnychuk D, Monette J, Abenhaim L. Determinants of initiation and suboptimal use of anti-ulcer medication: a study of the Quebec older population. J Am Geriatr Soc. 1997;45:853-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Morales Suárez-Varela MM, Pérez-Benajas MA, Girbes Pelechano VJ, Llopis-González A. Antacid (A02A) and antiulcer (A02B) drug prescription patterns: predicting factors, dosage and treatment duration. Eur J Epidemiol. 1998;14:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Bashford JN, Norwood J, Chapman SR. Why are patients prescribed proton pump inhibitors Retrospective analysis of link between morbidity and prescribing in the General Practice Research Database. BMJ. 1998;317:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Martin RM, Lim AG, Kerry SM, Hilton SR. Trends in prescribing H2-receptor antagonists and proton pump inhibitors in primary care. Aliment Pharmacol Ther. 1998;12:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Prach AT, McGilchrist MM, Murray FE, Johnston DA, MacDonald TM. Prescription of acid-suppressing drugs in relation to endoscopic diagnosis: a record-linkage study. Aliment Pharmacol Ther. 1999;13:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Boutet R, Wilcock M, MacKenzie I. Survey on repeat prescribing for acid suppression drugs in primary care in Cornwall and the Isles of Scilly. Aliment Pharmacol Ther. 1999;13:813-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Jones MI, Greenfield SM, Jowett S, Bradley CP, Seal R. Proton pump inhibitors: a study of GPs' prescribing. Fam Pract. 2001;18:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Bureau of National Health Insurance. 2001 National Health Insurance Annual Statistical Report. Taipei: Bureau of National Health Insurance 2002; . |
| 23. | Liu JY, Chen TJ, Hwang SJ. Concomitant prescription of non-steroidal anti-inflammatory drugs and antacids in the outpatient setting of a medical center in taiwan: a prescription database study. Eur J Clin Pharmacol. 2001;57:505-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Guidelines for ATC Classification and DDD Assignment, 3rd editor. Oslo: WHO Collaborating Centre for Drug Statistics Methodology 2000. . |
| 25. | OECD Health Data 2001. Paris: OECD (Organisation for Economic Co-operation and Development) 2001. . |
| 26. | Naunton M, Peterson GM, Bleasel MD. Overuse of proton pump inhibitors. J Clin Pharm Ther. 2000;25:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95:3118-3122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |