Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1352
Revised: August 1, 2002
Accepted: August 7, 2002
Published online: June 15, 2003
AIM: To construct and express a humanized M2 autoantigen trimer designated as BPO and to apply it in the diagnosis of primary biliary cirrhosis (PBC).
METHODS: cDNA fragments encoding M2-reactive epitopes of pyruvate dehydrogenase complex E2 (PDC-E2), branched chain 2-oxo-acid dehydrogenase complex E2 (BCOADC-E2) and 2-oxo-glutarate dehydrogenase complex E2 (OGDC-E2) were amplified with PCR using total RNA extracted from human peripheral mononuclear blood cells. The fragments were cloned into the plasmid vector pQE-30 and then transferred into E. coli M15 (pREP4) for expression, which was induced by isopropylthio-β-D-galactoside. The expressed recombinant BPO protein was demonstrated by SDS-PAGE, Western-blotting and Immunoabsorption test, its antigenic reactivity and specificity were identified with seven M---positive sera confirmed at Euroimmun Research Center (Germany). Using the purified BPO, M2 antibodies in sera from patients with PBC and other liver related diseases were detected with ELISA.
RESULTS: The expressed BPO was observed with both antigenic reactivity and specificity of M2 autoantigens. The determination of M2 antibodies by BPO with ELISA was more sensitive than using the Euroimmun's kit with the coefficients of variation less than 10% in both interassay and intraassay. With the newly established method, M2 antibodies were found in 100% (20/20) of patients with PBC. Six cases of liver disease with unknown etiology and 1 patient with drug induced liver injury had detectable levels of serum M2 antibodies. There were also 2 patients with autoimmune cholangitis and 1 with autoimmune hepatitis showing M2-antibody positive.
CONCLUSION: Compared with the routine immunofluorescence assay and commercially available assay kit using porcine heart mitochondrial protein as the antigen, the detection system established in the present study shows higher sensitivity and specificity and may be used as a powerful tool for the diagnosis of PBC.
- Citation: Jiang XH, Zhong RQ, Yu SQ, Hu Y, Li WW, Kong XT. Construction and expression of a humanized M2 autoantigen trimer and its application in the diagnosis of primary biliary cirrhosis. World J Gastroenterol 2003; 9(6): 1352-1355
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1352.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1352
Primary biliary cirrhosis (PBC) is a chronically progressive cholestatic liver disease with autoimmune basis. According to some reports, the incidence of this disease has been consistently increased in recent years[1-4]. One of the remarkable features of PBC is the appearance of high titre antimitochondrial antibodies (AMA) in the patient's sera. Generally, these antibodies are categorized into nine subgroups termed M1-M9 according to the antigens they recognize, in which only M2 antibodies are considered specific for PBC patients that are detectable years or decades before the clinical and histological diagnosis[5-11].
The major autoantigens recognized by M2 antibodies are the members of 2-oxo-acid dehydrogenase complex including pyruvate dehydrogenase complex E2 (PDC-E2), branched chain 2-oxo-acid dehydrogenase complex E2 (BCOADC-E2) and 2-oxo-glutarate dehydrogenase complex E2 (OGDC-E2), whose immunodominant epitopes have been mapped within lipoyl domains. Antibodies to these corresponding autoantigens have been reported in PBC patients with a positive rate of 95%, 53%-55% and 39%-88% respectively[6,12]. However, when all of these antibodies are determined simultaneously, the patients with PBC can be diagnosed as high as 92%-100%[13-16]. These facts suggest such a possibility that if there is a constructed antigen containing the specific immunodominant epitopes and the antibodies above can be detected synchronously, the diagnosis of PBC patients would be more specific, sensitive and convenient.
Therefore, we designed and constructed a M2 autoantigen trimer (BPO) expression vector, which could coexpress the immunodominant lipoyl domains of PDC-E2, BCOADC-E2 and OGDC-E2 from human origin, in an attempt to establish a more accurate and sensitive method with BPO to detect M2 antibodies in patients with PBC. Besides, because it has never been reported that M2 antibodies were found in other liver related diseases other than PBC[17-20], a survey to detect M2 antibodies under these circumstances with our constructed M2 autoantigen trimer was also included in the present study.
Eight groups of adult patients with both sexes who were treated in Shanghai Changzheng Hospital were enrolled in the present study. Group 1 consisted of 20 patients with PBC diagnosed on the criteria: the presence of AMA and at least one of the followings: (1) Elevation of serum alkaline phosphatase (ALP) and/or gamma glutamyl transpeptidase (γ-GT). (2) Liver biopsy with PBC characteristics[21]. Group 2 consisted of 5 patients with autoimmune hepatitis (AIH)[22]. Two patients diagnosed as autoimmune cholangitis (AIC) were included in group 3, and group 4 was composed of 18 patients diagnosed as liver disease with unknown etiology (LDUE) that was defined as lack of obvious causes including drug use, alcohol abuse, exposure to hepatotoxic medication or chemicals and virus infection. Group 5 consisted of 8 patients with drug induced liver injury (DILI). Group 6 enrolled 201 patients with other liver diseases (Post-viral hepatitis and liver cirrhosis, n = 153; Obstructive jaundice, n = 25; Acute hepatitis A, n = 15; Hepatic abscess, n = 3; Wilson’s disease, n = 1; Cardiac cirrhosis, n = 4). Thirty-three patients with various autoimmune diseases (AID) (Rheumatoid arthritis, n = 12; Systemic lupus erythmatosus, n = 12; Polymyositis, n = 4; Vasculitis, n = 3; Hashimoto's thyroiditis, n = 2) were included in group 7 and 1225 healthy volunteers taking a health checkup aged less than 28 served as the control. In the experiment, fasting serum from each patient was prepared with routine procedures and stored at -20 °C until further analysis.
Reverse transcriptase and PCR amplification system were purchased from Roche Company (USA). Restriction endonucleases and T4 DNA ligase were from New England Biolabs (USA). Plasmid vector pQE-30 and E.coli M15 (prep 4) were from Qiagen Company (Germany). Indirect immunofluorescence (IIF) test kit for AMA and Western-blotting kit for M2 antibodies were all from Euroimmun Company (Germany).
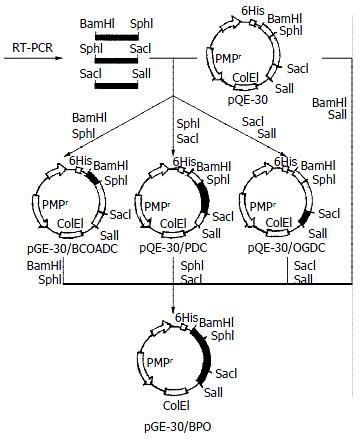
Recombinant plasmids were constructed as illustrated in Figure 1. Briefly, total RNA was extracted from human peripheral mononuclear blood cells. The objective cDNAs were synthesized by reverse transcriptase and used as the template to amplify the immunodominant epitopes of BCOADC-E2, PDC-E2 and OGDC-E2 with polymerase chain reaction. The PCR products were digested with relevant restriction endonuclease and purified cDNA fragments were inserted into the expression vector pQE-30 to form recombinant plasmids pQE-30/BCOADC-E2, pQE-30/PDC-E2, pQE-30/OGDC-E2 and pQE-30/BPO respectively. The pQE-30/BPO was then transferred into E. coli M15 (pREP4) and induced by isopropylthio-β-D-galactoside to express BPO protein, which was finally confirmed with SDS-PAGE, Western-blotting and Immunoabsorption test.
The antigenic reactivity and specificity of the recombinant BPO trimer were identified with seven M2-positive sera confirmed at Euroimmun Research Center (Germany) by immunoblotting using beef heart mitochondrial preparations.
The obtained recombinant BPO protein fused with the 6 × His affinity tag in the N-terminus was purified by Ni-NTA affinity chromatography under denaturing conditions. After renatured by removing denaturants slowly with dialysis, the BPO protein was used as the specific antigen to detect M2 antibodies with the routine procedures of ELISA. The coefficients of variation for this assay method, the mean OD ± SD for the control sera, as well as the critical OD value for the positive determination were respectively calculated or defined based on the experimental results. The measurements of M2 antibodies and AMA with Euroimmun's kits as a comparison of the present assay method were also simultaneously performed in the study.
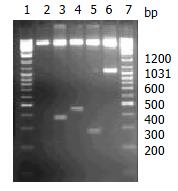
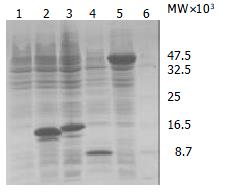
The segment analysis by restriction endonuclease digestion confirmed that inserted cDNA sequences in the constructed plasmids were completely consistent with that of the published data (Figure 2). The molecular mass of BPO protein was examined by SDS-PAGE in 15% polycrylamide gel, in which a specific 42 KD protein band was clearly visualized (Figure 3).
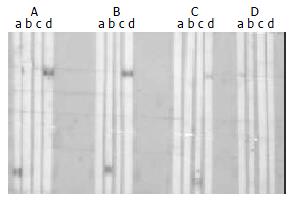
The expressed BPO protein could react with all of the seven M2-positive sera confirmed at Euroimmun Research Center (Germany) by immunoblotting using beef heart mitochondrial preparations, which identified the antigenic reactivity of the recombinant BPO trimer (Figure 4). When mixed beforehand with the lysates of E.coli expressing BPO overnight, the sera became M2-negative by Western-blotting, which confirmed the BPO specificity determined by the immunodominant epitopes of PDC-E2, BCOADC-E2 and OGDC-E 2.
The coefficients of variation for the detection of M2 antibodies by BPO with ELISA were less than 10% in both interassay and intraassay. The mean OD ± SD for the control sera was 0.073 ± 0.046. The critical OD value for positive was defined as ≥ 0.303 based on the mean control value + 5 SD.
In the patients with PBC who were AMA positive determined by IIF test kit, the positive rate of M2 antibodies detected by BPO with ELISA and Euroimmun's kit was 100% (20/20) and 80% (16/20) respectively (Table 1).
| Group | n | AMA positive | M2 - positive | AASLD's guideline (+) | |
| Euroimmun's kit | ELISA | ||||
| PBC | 20 | 20 | 16 | 20 | 20 |
| AIH | 5 | 0 | 0 | 1 | 0 |
| AIC | 2 | 0 | 1 | 2 | 0 |
| LDUE | 18 | 7 | 6 | 6 | 6 |
| DILI | 8 | 1 | 1 | 1 | 1 |
| Other liver diseases | 201 | ND | ND | 0 | ND |
| AID | 33 | 3 | 0 | 0 | 0 |
| Control | 1225 | ND | ND | 0 | ND |
Seven patients with liver disease of unknown etiology were all AMA positive; However, only one was M2 antibody negative and didn't agree with the guideline by the American Association for the Study of Liver Diseases (AASLD), and his plasma ALP and γ-GT were in normal range but alanine aminotransferase was elevated (120 U/L). The other 6 patients with M2 antibody positive had no specific symptoms except the unexplained elevation of serum ALP (187-1525 U/L) and γ-GT (88-2685 U/L).
One patient with drug induced liver injury was demonstrated as M2 antibody positive by both ELISA and Euroimmun's kit, whose additional laboratory data were as follows: AMA positive, antinuclear antibodies positive, ALP 153 U/L, γ-GT 321 U/L; alanine aminotransferase 281 U/L, aspartate transaminase 225 U/L, total bilirubin 25 mmol/L (normal < 18 μmol/L). This patient suffered from lymphatic tuberculosis and had taken rifampisin for one year before the onset of liver disease. He was in agreement with the AASLD's guideline.
It was noteworthy that the sera from 1 AIH and 2 AIC patients with AMA negative had detectable M2 antibody by BPO with ELISA, while they were M2 antibody negative with the Euroimmun's kit. The prominent elevation of plasma ALP and γ-GT was observed in all of the three patients.
No M2 antibody positive sera were found in control, other liver disease and the AID group by BPO with ELISA.
In the guideline by AASLD in 2000 and the standards by other researchers, AMA has long been used as an important marker for the primary biliary cirrhosis[21,23]; however, only M2 antibodies are considered as specific for the PBC diagnosis. Other AMA sub-types have been found in drug-induced disorders, cardiomyopathies, systemic lupus erythmatosus, rheumatoid arthritis, tuberculosis, syphilis and hepatitis C, indicating the nonspecific nature of AMA in the diagnosis of PBC[24]. Besides, there were about 5%-17% of the patients with biochemical and histological features compatible with PBC not having detectable AMA with the IIF method[25-34]. To get better diagnostic results, approaches to detect M2 antibodies by ELISA or Western-blotting using recombinant antigen of PDC-E2, BCOADC-E2 and OGDC-E2 have been reported in several literatures[15-17].
In 2001, Miyakawa and his coworkers[35] developed a new ELISA for the detection of M2 antibody using porcine heart mitochondrial protein as the antigen. The sensitivity of this method was only 78%, despite the specificity was 100%. In the present study, we employed BPO as the antigen to determine M2 antibodies with ELISA, which was more sensitive than the Euroimmun's kit. The reason for this was partially because the antigen used in our approach was derived from human sources instead of that from porcine used in Euroimmun's kit. The antigen heterogeneity might affect the assay results[36]. Furthermore, the three major autoantigens, BCOADC-E2, PDC-E2 and OGDC-E2, with no cross-reactivity between, were constructed together as a trimer by molecular biological techniques, which could provide more positive chance for the detection of M2 antibodies. Therefore, the use of this recombinant molecule offered a rapid, simple and sensitive ELISA for the immunodiagnosis of PBC.
According to the investigation by James and his associates[3], the incidence of PBC has been increased in recent years. In northern England, the prevalence of PBC from 201.9 per 106 adults and 541.4 per 106 women over 40 in 1987 rose to 334.6 and 939.8 respectively in 1994. Owing to the lack of sensitive diagnostic methods, there have no reliable data related to the epidemiology of PBC in China so far and more seriously, clinical doctors have not yet paid appropriate attention to this disease. We checked 10 patients with liver cirrhosis hospitalized in January, February and April in 2000 whose serum immunological variables showed no signs of viral infection, and the reason for liver cirrhosis seemed unclear. However, 7 of the 10 patients were found M2 antibody positive by the detailed studies at the Euroimmun Research Center (Germany). In the past six months since we detected M2 antibody by BPO with ELISA for the PBC diagnosis, over 120 patients sera have been examined, in which 69 demonstrated M2 antibody positive and 30 cases with comparatively complete clinical data listed in this paper. Our recent research and the related domestic reports in 2001 indicate that PBC is probably not so rare in China as it has been thought[4,37].
Edited by Zhu L
| 1. | James OF, Bhopal R, Howel D, Gray J, Burt AD, Metcalf JV. Primary biliary cirrhosis once rare, now common in the United Kingdom. Hepatology. 1999;30:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 155] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Metcalf J, James O. The geoepidemiology of primary biliary cirrhosis. Semin Liver Dis. 1997;17:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Metcalf JV, Bhopal RS, Gray J, Howel D, James OF. Incidence and prevalence of primary biliary cirrhosis in the city of Newcastle upon Tyne, England. Int J Epidemiol. 1997;26:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Medina J, Jones EA, García-Monzón C, Moreno-Otero R. Immunopathogenesis of cholestatic autoimmune liver diseases. Eur J Clin Invest. 2001;31:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Heathcote EJ. Evidence-based therapy of primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 1999;11:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Joplin RE, Neuberger JM. Immunopathology of primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 1999;11:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Metcalf JV, Mitchison HC, Palmer JM, Jones DE, Bassendine MF, James OF. Natural history of early primary biliary cirrhosis. Lancet. 1996;348:1399-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 273] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Kisand KE, Metsküla K, Kisand KV, Kivik T, Gershwin ME, Uibo R. The follow-up of asymptomatic persons with antibodies to pyruvate dehydrogenase in adult population samples. J Gastroenterol. 2001;36:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Koizumi H, Onozuka Y, Shibata M, Sano K, Ooshima Y, Morizane T, Ueno Y. [Positive rate of anti-mitochondrial antibody in Japanese corporate workers]. Rinsho Byori. 2000;48:966-970. [PubMed] |
| 10. | Turchany JM, Uibo R, Kivik T, Van de Water J, Prindiville T, Coppel RL, Gershwin ME. A study of antimitochondrial antibodies in a random population in Estonia. Am J Gastroenterol. 1997;92:124-126. [PubMed] |
| 11. | Nakano T, Inoue K, Hirohara J, Arita S, Higuchi K, Omata M, Toda G. Long-term prognosis of primary biliary cirrhosis (PBC) in Japan and analysis of the factors of stage progression in asymptomatic PBC (a-PBC). Hepatol Res. 2002;22:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Migliaccio C, Van de Water J, Ansari AA, Kaplan MM, Coppel RL, Lam KS, Thompson RK, Stevenson F, Gershwin ME. Heterogeneous response of antimitochondrial autoantibodies and bile duct apical staining monoclonal antibodies to pyruvate dehydrogenase complex E2: the molecule versus the mimic. Hepatology. 2001;33:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Kitami N, Komada T, Ishii H, Shimizu H, Adachi H, Yamaguchi Y, Kitamura T, Oide H, Miyazaki A, Ishikawa M. Immunological study of anti-M2 in antimitochondrial antibody-negative primary biliary cirrhosis. Intern Med. 1995;34:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Jones DE. Autoantigens in primary biliary cirrhosis. J Clin Pathol. 2000;53:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Miyakawa H, Tanaka A, Kikuchi K, Matsushita M, Kitazawa E, Kawaguchi N, Fujikawa H, Gershwin ME. Detection of antimitochondrial autoantibodies in immunofluorescent AMA-negative patients with primary biliary cirrhosis using recombinant autoantigens. Hepatology. 2001;34:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Kitami N, Ishii H, Shimizu H, Adachi H, Komada T, Mikami H, Yokoi Y, Sato N. Immunoreactivity to M2 proteins in antimitochondrial antibody-negative patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 1994;9:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Jensen WA, Jois JA, Murphy P, De Giorgio J, Brown B, Rowley MJ, Mackay IR. Automated enzymatic mitochondrial antibody assay for the diagnosis of primary biliary cirrhosis. Clin Chem Lab Med. 2000;38:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Leung PS, Van de Water J, Coppel RL, Nakanuma Y, Munoz S, Gershwin ME. Molecular aspects and the pathological basis of primary biliary cirrhosis. J Autoimmun. 1996;9:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Strassburg CP, Manns MP. Autoimmune tests in primary biliary cirrhosis. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:585-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Quaranta S, Shulman H, Ahmed A, Shoenfeld Y, Peter J, McDonald GB, Van de Water J, Coppel R, Ostlund C, Worman HJ. Autoantibodies in human chronic graft-versus-host disease after hematopoietic cell transplantation. Clin Immunol. 1999;91:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Parikh-Patel A, Gold EB, Worman H, Krivy KE, Gershwin ME. Risk factors for primary biliary cirrhosis in a cohort of patients from the united states. Hepatology. 2001;33:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Ma X, Qiu DK. Relationship between autoimmune hepatitis and HLA-DR4 and DRbeta allelic sequences in the third hypervariable region in Chinese. World J Gastroenterol. 2001;7:718-721. [PubMed] |
| 23. | Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 275] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 24. | Strassburg CP, Jaeckel E, Manns MP. Anti-mitochondrial antibodies and other immunological tests in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 1999;11:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Michieletti P, Wanless IR, Katz A, Scheuer PJ, Yeaman SJ, Bassendine MF, Palmer JM, Heathcote EJ. Antimitochondrial antibody negative primary biliary cirrhosis: a distinct syndrome of autoimmune cholangitis. Gut. 1994;35:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 141] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Lacerda MA, Ludwig J, Dickson ER, Jorgensen RA, Lindor KD. Antimitochondrial antibody-negative primary biliary cirrhosis. Am J Gastroenterol. 1995;90:247-249. [PubMed] |
| 27. | Heathcote J. Autoimmune cholangitis. Gut. 1997;40:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Ikuno N, Scealy M, Davies JM, Whittingham SF, Omagari K, Mackay IR, Rowley MJ. A comparative study of antibody expressions in primary biliary cirrhosis and autoimmune cholangitis using phage display. Hepatology. 2001;34:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Kinoshita H, Omagari K, Whittingham S, Kato Y, Ishibashi H, Sugi K, Yano M, Kohno S, Nakanuma Y, Penner E. Autoimmune cholangitis and primary biliary cirrhosis--an autoimmune enigma. Liver. 1999;19:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Invernizzi P, Crosignani A, Battezzati PM, Covini G, De Valle G, Larghi A, Zuin M, Podda M. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Hepatology. 1997;25:1090-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 186] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Kaserer K, Exner M, Mosberger I, Penner E, Wrba F. Characterization of the inflammatory infiltrate in autoimmune cholangitis. A morphological and immunhistochemical study. Virchows Arch. 1998;432:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Mayo MJ, Lipsky PE, Miller SN, Stastny P, Combes B. Similar T-cell oligoclonality in antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Dig Dis Sci. 2001;46:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Fujioka S, Yamamoto K, Okamoto R, Miyake M, Ujike K, Shimada N, Terada R, Miyake Y, Nakajima H, Piao CY. Laparoscopic features of primary biliary cirrhosis in AMA-positive and AMA-negative patients. Endoscopy. 2002;34:318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Nakajima M, Shimizu H, Miyazaki A, Watanabe S, Kitami N, Sato N. Detection of IgA, IgM, and IgG subclasses of anti-M2 antibody by immunoblotting in autoimmune cholangitis: is autoimmune cholangitis an early stage of primary biliary cirrhosis. J Gastroenterol. 1999;34:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Miyakawa H, Kikuchi K, Jong-Hon K, Kawaguchi N, Yajima R, Ito Y, Maekubo H. High sensitivity of a novel ELISA for anti-M2 in primary biliary cirrhosis. J Gastroenterol. 2001;36:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Miyakawa H, Kawaguchi N, Kikuchi K, Fujikawa H, Kitazawa E, Matsushita M. Definition of antigen specificity for antimitochondrial proteins detected by Western blotting using native mitochondrial proteins in primary biliary cirrhosis. Hepatol Res. 2001;21:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Zhang F, Jia J, Wang B, Qian L, Yin S, Wang Y, Cui Y, You H, Ma H, Wang H. [Clinical characteristics of primary biliary cirrhosis: a report of 45 cases]. Zhonghua Neike Zazhi. 2002;41:163-167. [PubMed] |