Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1327
Revised: December 4, 2002
Accepted: December 20, 2002
Published online: June 15, 2003
AIM: To evaluate the protective effect and mechanism of glutamine on the intestinal barrier function in total parenteral nutrition (TPN) rats with trauma or endotoxemia.
METHODS: To perform prospective, randomized and controlled animal experimentation of rats with surgical trauma, TPN and endotoxemia, thirty-four male, adult Sprague Dawley rats were divided into four groups: control group (n = 8), TPN group (n = 9), trauma and endotoxemia group (LPS, n = 8) and trauma plus endotoxemia supplemented with glutamine in TPN solution group (Gln.group, n = 9). All groups except the control group were given TPN solutions in 7-day experimental period. For Gln group, 1000 mg/kg/d of glutamine was added to TPN solution during day 1-6. On the 7th day all the animals were gavaged with lactulose (66 mg) and mannitol (50 mg) in 2 mL of normal saline. Then 24 h urine with preservative was collected and kept at -20 °C. On day 8, under intra-peritoneal anesthesia using 100 mg/kg ketamin, the intestine, liver, mesenteric lymph nodes and blood were taken for examination.
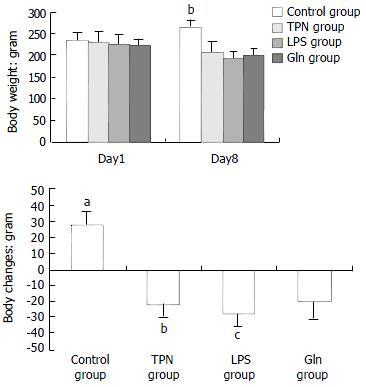
RESULTS: The body weight of LPS group decreased most among the four groups. The structure of small intestinal mucosa in TPN group, LPS group and Gln group showed impairments of different degrees, and the damage of small intestinal mucosa in Gln group was remarkably alleviated. The concentrations of interleukins in small intestine mucosa were lower (for IL-4 and IL-6) or the lowest (IL-10) in Gln group. The IgA level in the blood plasma and the mucosa of Gln group was the highest among all of the groups. The urine lactulose/mannitol test showed that the intestinal permeability in LPS group was lower than that in TPN group (P < 0.001), but there was no difference between LPS group and Gln group. The rate of bacterial translocation in Gln group was lower than that in LPS group (P < 0.02).
CONCLUSION: Prophylactic treatment with glutamine could minimize the increments of intestinal permeability and bacterial translocation caused by trauma and endotoxemia in rats treated with TPN.
- Citation: Ding LA, Li JS. Effects of glutamine on intestinal permeability and bacterial translocation in TPN-rats with endotoxemia. World J Gastroenterol 2003; 9(6): 1327-1332
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1327
Trauma, burn, infection, surgical operations and the use of intravenous alimentation over a long period can damage the barrier function of the intestine, leading to atrophy of the intestinal mucosa, increase of mucosal permeability, decrease of immunity, and occurrence of bacterial/endotoxin translocation[1-5]. If the causes could not be removed or the stress is too severe when the intestinal barrier is protected, the damage of the intestinal mucosa would become more severe and multiple organ dysfunction syndrome (MODS) would ensue. These adverse outcomes are commonly seen clinically and methods of their prevention need to be investigated. Therefore we studied the mechanism of protective effect of glutamine on the intestinal barrier function in TPN rats with trauma and endotoxemia.
Adult, healthy male Sprague-Dawley rats, with body weight of 150-180 g (supplied by Shanghai Experimental Animal Center, the Chinese Academy of Sciences) were used. The rats were fed for over a week in our lab for their adaptation and then were put into metabolic cages for 5-7 d. The temperature in the animal rooms was 17-21 °C with 60% humidity and illumination of 12 h/d (6:00-18:00). During the adaptation period all rats were fed with regular rat chow and tap water ad libitum. When the rat's weight reached 200-300 g, thirty-four rats were chosen randomly and divided into four groups: 1. control group (n = 8), fed rat chow and tap water freely; 2. TPN group (n = 9), infused with a whole nutrients solution through a central venous catheter, and with drinking water ad libitum; 3. LPS group (trauma + TPN + endotoxemia, n = 8), in which an exploratory laparotomy and central venous catheterization served as the trauma. After this, TPN was their sole nutrition source plus drinking water ad libitum. On the 7th day 5 mg/kg of lipopolysaccharide (LPS) was injected intraperitoneally; 4. Gln group, (trauma + TPN + endotoxemia + glutamine, n = 9) in which all the treatments were the same as LPS group but on days 2-6, 1000 mg/kg/d glutamine (Dipeptiven) was added to TPN solution. All groups except the control one received isonitrogenous, isocaloric and isovolumic TPN solution during the 7-day period. All the protocols and procedures were approved by our University Committee of Animal Experiment Administration.
TPN ingredients 11.4% compound amino acids injection (Novamin), 20% mid-long chain fat emulsion (Lipovenoes MCT), Dipeptiven (alanyl-glutamine dipeptide solution), multivitamin mixture (Soluvit, Vitalipid) and a trace element mixture (Addamel), all from Sino-Sweden and Fresenius Pharmaceutical Corp. LTD.
Chemicals and reagents Lipopolysaccharide (LPS, from E Coli, 055: B5) was purchased from Sigma Co.; IL-4 from Diaclone Co.of France; IL-6 and IL-10, from Biosource Co., USA; and IgA, from Bethyl Co.USA.
Operation procedures Under anaesthesia of 100 mg/kg of ketamin injected into the animals intraperitoneally, the TPN model was constructed and a rotary transfusion apparatus was used for TPN infusion[6,7]. For surgical trauma, the animal’s abdomen after shaving and incision (4 cm in length) was exposed and examined from the epigastrium to the pelvic cavity. The incision was sutured in double layers with silk suture No. 1 and operation was performed aseptically.
TPN solution The rats were put in the metabolic cages after surgical recovery. Each rat received 230 cal/kg body wt of calories and 1.42 g nitrogen/kg each day in 50 mL of TPN solution. The ratio of glucose to lipid in this solution was 2:1, and nonprotein calorie to nitrogen (kcal/g), 137:1. Multivitamins, electrolytes, trace elements and 500 units of heparin were also included in the TPN solution. All the nutrient solutions were prepared under aseptic conditions daily and the infusion was done with an injecting micropump continuously and uniformly during 24 h each day. The TPN infusion was started immediately after recovery from the laparotomy. On the first and last days of the experiment, each rat was given half of the total calories without any changes of other TPN ingredients.
Inducing endotoxemia On the 7th day of the experiment, 5 mg/kg of LPS in 5 mL of sterile distilled water was injected into the animals peritoneal cavity to cause a septic state.
Lactulose and mannitol solution gavage On the 7th day of the experiment, 66 mg of lactulose and 50 mg of mannitol dissolved in 2 mL of normal saline were gavaged. Twenty-four hour urine was collected, with the volume recorded and 0.2 mL of mercury salicylosulfide added. Then 5 mL of the urine specimen was stored at -20 °C until measured.
Twenty-four hours after gavaging with lactulose and mannitol and injecting endotoxin, 100 mg/kg of ketamine was injected intraperitoneally as an anesthetic. After the laparotomy was done, tissue and blood samples were collected and examinations were performed.
Bacteriological test 0.5 mL of blood from the portal vein was drawn for culture. One gram of anterior lobe liver tissue and about 0.5 g mesenteric lymph nodes were excised. Each sample was put in a tissue homogenizer and an equivalent amount of normal saline was added before they were homogenized. The specimens were sent to microbiological laboratory for aerobic culture and bacterial identification by morphological and biochemical examinations.
Bacterial culture (1) 10 μL homogenates of the lymph nodes and liver homogenates were separately taken and put on blood agar plates. Another 10 μL lymph nodes and liver homogenates were mixed with 10 mL saline for a dilution and the diluted samples were inoculated also on blood agar plates. (2) 0.5 mL of portal vein blood was inoculated into 4.5 mL of common broth for bacterial enrichment of 16 h, then 20 μL of the enrichment solution was inoculated on blood agar plates. (3) The cultured media were put in a CO2 incubator at 35 °C for 24-48 h. If there was no bacterial growth, they would be regarded as negative, but if there was growth, it would be further identified.
Identification of bacteria First, Gram stained smears were made to determine whether they were coccus or bacillus and G+ or G-. Second, biochemical and serological identifications were made using standard and routine methods.
Preparation and examination of small intestine specimens The whole small intestine below the Treitz ligament was excised and immediately placed in icy 0.9% saline. The intestine was opened longitudinally and the contents of the intestine were washed out with icy saline. Two cm of proximal jejunum and distal ileum was cut and put into 10% neutral formalin solution promptly and sent to be examined histologically.
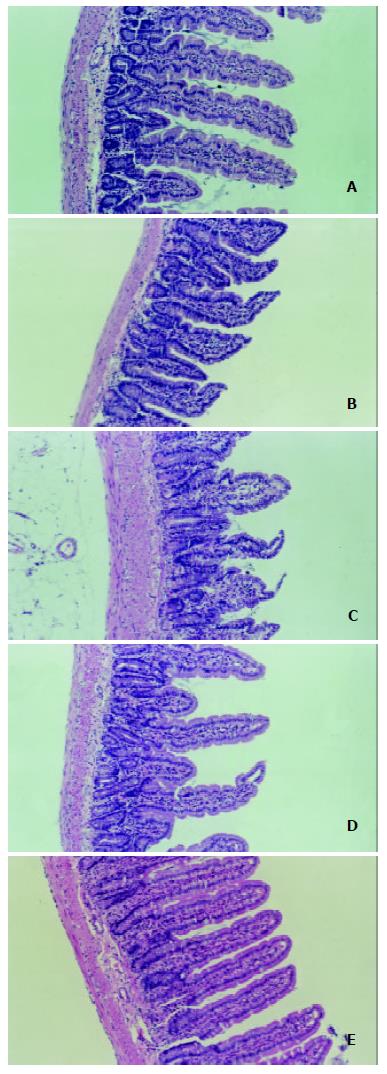
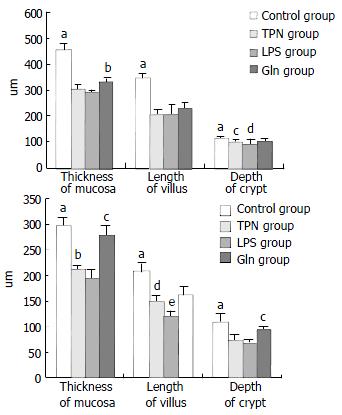
Histological examination of intestinal mucosa Specimens were embedded in paraffin, 4 μm sections made and stained with H.E., analyzed with an HPIAS-1000 Multimedia Color Analysis System. Three low power (10 × 10) fields in each slide were read. The length of 5 villi, the depth of 5 crypts and the thickness of the mucosa at 5 sites were read and analyzed. The average value was calculated and documented. All the manipulations were done blindly by two experienced pathologists.
Bloodletting and animal execution Three mL of blood from the right ventricle was drawn and 1250 u of heparin was added. The blood was centrifuged and the serum was stored at -70 °C. Then the animals were sacrificed by exsanguination.
Lactulose/mannitol test The lactulose and mannitol concentrations in the preserved urine sample were measured by a high-performance liquid chromatograph (Waters Co., USA). The ion-exchange column used was bought from Transgenomic Co., USA. The ratio between the two sugars was then calculated.
Identification of the interleukins About 5 cm intestine segments from the upper, middle and lower paste were resected and then the surfaces of the mucosa were dried with cotton swabs. The mucous membrane of the icy specimens was scraped, weighed and divided into two equal parts. They were immediately put into liquid nitrogen and then stored at -70 °C. For the tests, the specimens were melted to room temperature, and 1 mL normal saline was added before homogenates were made. The homogenates were then centrifuged for 15 min at 4 °C and then IL-4, IL-6 and IL-10 in the supernatants were measured with ELISA method described by Kudsk[8]. The results were described as pg/g of mucosal tissue of the small intestine.
Determination of IgA The frozen samples of blood plasma and the mucosa of the small intestine were melted to room temperature and the concentration of IgA in them was measured by ELISA method. The results were shown as IgA μg/mL of blood plasma and IgA μg/g of small intestinal mucosa.
All the values were expressed as the mean ± SD. One-way ANOVA was used to check the differences between them. ChiSquare test was used to check the differences of bacterial translocation rates between the groups. When P was less than 0.05, the difference was considered statistically significant. The degree of correlation was described using Pearson Correlation coefficient. Software SPSS10.0 was used in all statistical analyses.
The body weight changes of the animals in each group are illustrated in Figure 1. At the beginning of the experiment there was no difference in the body weight of the animals among the groups. At the end of the experiment, the body weight decrease was greatest in the animals of LPS group.
The degree of damage of villi and crypts and the thinning of mucous membrane in jejunum and ileum were most significant in LPS group among all groups (Figure 2 and Figure 3).
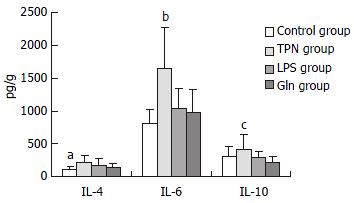
The concentrations of IL-4 and IL-6 in Gln group were the lowest among these groups except control group. IL-10 level in Gln group was also the lowest among the four groups, and it was significantly lower than that in TPN group (P < 0.01, Figure 4).
IgA levels in blood plasma in Gln group were the highest among the four groups, and so was that in small intestinal mucosa. There was a positive correlation for IgAs level between those in plasma and small intestinal mucosa (r = 0.961, P < 0.04, Table 1).
There was no significant correlation between IgA level in plasma and mucosa with the level of interleukins in mucosal tissue of small intestine (Table 2).
| IgA of blood plasma | IgA of mucous membrane | |||||
| IL-4 | IL-6 | IL-10 | IL-4 | IL-6 | IL-10 | |
| r value | -0.562 | -0.584 | 0.744 | -0.614 | -0.373 | -0.396 |
| P value | 0.438 | 0.416 | 0.256 | 0.386 | 0.627 | 0.604 |
| Correlationa | no | no | no | no | no | no |
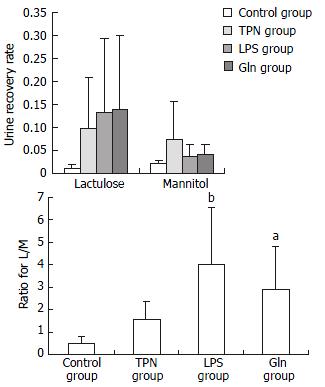
The recovery rates of lactulose and mannitol in the urine of TPN group, LPS group and Gln group were increased. The ratio of L/M was the biggest in LPS group, and the value of the ratio in LPS group was not different statistically from that in Gln group (Figure 5).
The results of bacterial culture were labeled as positive when the CFU found per gram of tissue (or mL of blood) was more than or equivalent to 103[9] . In LPS group the rate of bacterial translocation was the highest. The second highest rate was seen in TPN group. The logarithm of the number of translocated bacteria correlated positively with the bacterial translocation rate (Table 3). The bacteria translocated, in order of frequency, were proteus, E. coli, enterococcus and other Gram negative bacteria. One or two, even three bacteria were usually recovered from the same organ when translocation was present[6,9].
Damage of intestinal barrier function and the resulting bacterial translocation and endotoxemia caused by stress was well documented in the literature[1-4,10-14]. How to protect this barrier function and prevent bacterial translocation and toxemia during stress are important research topics[15-17].
Animal experiments showed some good results in protecting intestinal barrier function and decreasing bacterial translocation, toxemia and enterogenous infection by various measures[6,8,9,15,17-22,30]. Glutamine has many biological functions[23,24]. It comprises more than 50 percent of the body's free amino acid pool and is a precursor for synthesis of nucleic acids and glutathione. It is the main fuel for rapid proliferating and dividing cells such as enterocytes, lymphocytes, and other immunocytes etc. It can promote hyperplasia of the epithelial cells of ileum and colon[25]. The structure and function of small intestinal mucosa are maintained and the increment of intestinal permeability is reduced when glutamine is supplemented to animals fed parenterally[26-28]. Besides, glutamine could enhance body's immunity through immune modulation[8,24,29]. Results from a series of experiments and clinical investigations indicate that supplementation with glutamine parenterally and/or enterally has improved the gut barrier function and body's immunity when used in human and animals[13,17,23,29-35]. It has been generally accepted that glutamine could maintain intestinal barrier function through the improvement of architecture of small intestinal mucosa. Formerly, researches along this line usually paid attention to only one aspect of the functions of the intestinal barrier. For this reason, no interrelationship among the permeability and immunity of intestinal mucosa and bacterial translocation was discussed or fully explained[6,11,30].
It is known that the cause of the damage of intestinal permeability in animals fed with TPN is mainly the atrophy of intestinal mucous membrane[11,34]. The mechanism of the increase of intestinal permeability caused by endotoxin is very complicated. It may relate to many inflammation mediators such as cytokines, vasoactive amines and oxygen free radicals[16,36]. The problem needs further investigation. Our experiment showed that injection of LPS intraperitoneally to rats indeed led to impairment of gut barrier function and an increase in bacterial translocation. Although there were no differences in body weight change when comparing that in glutamine-supplemented group with those in TPN group and LPS group, the damage of architecture of small intestinal mucosa in the former, especially in mucous membrane of ileum, was greatly alleviated than those in the latter two groups.
Glutamine has a visible effect on the secretion of interleukins in mucous membrane of small intestine. The three interleukins in small intestinal mucosa in glutamine group were lower (for IL-4 and IL-6) or lowest (IL-10) than those in other groups of our experiment. There are different opinions about the effects of interleukins on intestinal barrier function. Some authors mentioned that intestinal IL-4 and IL-10 were important in maintaining IgA concentration in the respiratory and alimentary tracts and in the blood[8]. In the present experiment, we have not found a correlation between IgA level and IL-4 and IL-10 levels in intestinal mucosa. It has been considered that type II cytokines such as IL-4 and IL-10 have an anti-inflammatory function and then it could alleviate tissue and cell damage caused inflammatory mediators and type I cytokines such as IL-6[37-39]. But there was a report that held a completely different opinion[40]. We could see from our experiment that glutamine could reduce secretion of interleukins, and it seems that the lower interleukin concentrations are beneficial in reducing the damage of intestinal permeability[40].
It is known that the digestive tract is the largest immune organ in human body and intestinal mucosal IgA is the first defense line of intestine immunity barrier. This has an important function in preventing bacterial adherence and translocation within intestinal lumen. In our experiment, the IgA levels in blood and intestinal mucosa in glutamine group were the highest among all groups. There was statistically significant difference between the mucosal IgA levels in glutamine group and those in the control and TPN groups. This result meant that the alleviation of bacterial translocation rate in animals supplemented with glutamine correlated with the increase of IgA secretion in mucous membrane of the small intestine. There was a positive correlation between IgA levels in mucous membrane and blood plasma. It confirmed that the intestine is a vital organ for IgA secretion[32].
Many changes of the immunological indicators in our experiment did not reach a statistically significant level. The reason for this might be that our experiment and observation period ended at the time when the peak of stress reaction had just passed and the animals had no sufficient recovery time[12]. In this experiment we have made a model in which the animal is injured by laparotomy and parenteral nutrition, and endotoxemia is caused by an injection with a higher dosage of LPS intraperitoneally 6 d after the injury. The protective mechanism of glutamine on gut barrier was investigated. We found that the model simulated well the conditions of the clinical infectious complications.
In summary, using 1000 mg/kg/d glutamine parenterally can alleviate the atrophy and impairment of the mucous membrane of small intestine in rats. It can also increase the concentration of IgA and decrease concentrations of IL-4, IL-6 and IL-10 that are secreted by the mucous membrane of small intestine. Thus, the immune function of the small intestinal mucosa was modulated and the damage of gut barrier caused by trauma and endotoxemia was alleviated. The rate of bacterial translocation was also decreased. Changes of intestinal permeability measured with the dual sugar test did not completely correlated with alterations of gut barrier function[41].
We would like to thank Prof. Shao Haifeng and Prof. Xu Genbao for their technical guidance and help; we are grateful to the staff of Animal Lab. and Institute of General Surgery for their selfless work and support in this investigation.
Edited by Wu XN
| 1. | Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 424] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 291] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Wilmore DW, Smith RJ, O'Dwyer ST, Jacobs DO, Ziegler TR, Wang XD. The gut: a central organ after surgical stress. Surgery. 1988;104:917-923. [PubMed] |
| 4. | van der Hulst RR, von Meyenfeldt MF, van Kreel BK, Thunnissen FB, Brummer RJ, Arends JW, Soeters PB. Gut permeability, intestinal morphology, and nutritional depletion. Nutrition. 1998;14:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 583] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Eizaguirre I, Aldazabal P, Barrena MJ, Garcia-Arenzana JM, Ariz C, Candelas S, Tovar JA. Effect of growth hormone on bacterial translocation in experimental short-bowel syndrome. Pediatr Surg Int. 1999;15:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Zhou X, Li YX, Li N, Li JS. Effect of bowel rehabilitative therapy on structural adaptation of remnant small intestine: animal experiment. World J Gastroenterol. 2001;7:66-73. [PubMed] |
| 8. | Kudsk KA, Wu Y, Fukatsu K, Zarzaur BL, Johnson CD, Wang R, Hanna MK. Glutamine-enriched total parenteral nutrition maintains intestinal interleukin-4 and mucosal immunoglobulin A levels. JPEN J Parenter Enteral Nutr. 2000;24:270-274; discussion 270-274;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Huang KF, Chung DH, Herndon DN. Insulinlike growth factor 1 (IGF-1) reduces gut atrophy and bacterial translocation after severe burn injury. Arch Surg. 1993;128:47-53; discussion 53-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Naaber P, Smidt I, Tamme K, Liigant A, Tapfer H, Mikelsaar M, Talvik R. Translocation of indigenous microflora in an experimental model of sepsis. J Med Microbiol. 2000;49:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | MacFie J. Enteral versus parenteral nutrition: the significance of bacterial translocation and gut-barrier function. Nutrition. 2000;16:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Deitch EA, Ma WJ, Ma L, Berg RD, Specian RD. Protein malnutrition predisposes to inflammatory-induced gut-origin septic states. Ann Surg. 1990;211:560-567; discussion 560-567;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | MacFie J, O'Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 290] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Reynolds JV, Murchan P, Leonard N, Clarke P, Keane FB, Tanner WA. Gut barrier failure in experimental obstructive jaundice. J Surg Res. 1996;62:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Chen K, Okuma T, Okamura K, Tabira Y, Kaneko H, Miyauchi Y. Insulin-like growth factor-I prevents gut atrophy and maintains intestinal integrity in septic rats. JPEN J Parenter Enteral Nutr. 1995;19:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Mishima S, Xu D, Deitch EA. Increase in endotoxin-induced mucosal permeability is related to increased nitric oxide synthase activity using the Ussing chamber. Crit Care Med. 1999;27:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Fukushima R, Saito H, Inoue T, Fukatsu K, Inaba T, Han I, Furukawa S, Lin MT, Muto T. Prophylactic treatment with growth hormone and insulin-like growth factor I improve systemic bacterial clearance and survival in a murine model of burn-induced gut-derived sepsis. Burns. 1999;25:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Vázquez I, Gómez-de-Segura IA, Grande AG, Escribano A, González-Gancedo P, Gómez A, Díez R, De Miguel E. Protective effect of enriched diet plus growth hormone administration on radiation-induced intestinal injury and on its evolutionary pattern in the rat. Dig Dis Sci. 1999;44:2350-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Ziegler TR, Leader LM, Jonas CR, Griffith DP. Adjunctive therapies in nutritional support. Nutrition. 1997;13:64S-72S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Chen DL, Wang WZ, Wang JY. Epidermal growth factor prevents gut atrophy and maintains intestinal integrity in rats with acute pancreatitis. World J Gastroenterol. 2000;6:762-765. [PubMed] |
| 21. | Gu Y, Wu ZH. The anabolic effects of recombinant human growth hormone and glutamine on parenterally fed, short bowel rats. World J Gastroenterol. 2002;8:752-757. [PubMed] |
| 22. | Zhou X, Li N, Li JS. Growth hormone stimulates remnant small bowel epithelial cell proliferation. World J Gastroenterol. 2000;6:909-913. [PubMed] |
| 23. | Heys SD, Ashkanani F. Glutamine. Br J Surg. 1999;86:289-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Pichard C, Kudsk KA. From nutrition support to pharmacologic nutrition in the ICU. Germany, Springer. 2000;30-31. [DOI] [Full Text] |
| 25. | Scheppach W, Loges C, Bartram P, Christl SU, Richter F, Dusel G, Stehle P, Fuerst P, Kasper H. Effect of free glutamine and alanyl-glutamine dipeptide on mucosal proliferation of the human ileum and colon. Gastroenterology. 1994;107:429-434. [PubMed] |
| 26. | van der Hulst RR, van Kreel BK, von Meyenfeldt MF, Brummer RJ, Arends JW, Deutz NE, Soeters PB. Glutamine and the preservation of gut integrity. Lancet. 1993;341:1363-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 446] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 27. | Khan J, Iiboshi Y, Cui L, Wasa M, Sando K, Takagi Y, Okada A. Alanyl-glutamine-supplemented parenteral nutrition increases luminal mucus gel and decreases permeability in the rat small intestine. JPEN J Parenter Enteral Nutr. 1999;23:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Li JY, Lu Y, Hu S, Sun D, Yao YM. Preventive effect of glutamine on intestinal barrier dysfunction induced by severe trauma. World J Gastroenterol. 2002;8:168-171. [PubMed] |
| 29. | Burke DJ, Alverdy JC, Aoys E, Moss GS. Glutamine-supplemented total parenteral nutrition improves gut immune function. Arch Surg. 1989;124:1396-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 204] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Scopa CD, Koureleas S, Tsamandas AC, Spiliopoulou I, Alexandrides T, Filos KS, Vagianos CE. Beneficial effects of growth hormone and insulin-like growth factor I on intestinal bacterial translocation, endotoxemia, and apoptosis in experimentally jaundiced rats. J Am Coll Surg. 2000;190:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Juby LD, Rothwell J, Axon AT. Lactulose/mannitol test: an ideal screen for celiac disease. Gastroenterology. 1989;96:79-85. [PubMed] |
| 32. | Hulsewé KW, van Acker BA, von Meyenfeldt MF, Soeters PB. Nutritional depletion and dietary manipulation: effects on the immune response. World J Surg. 1999;23:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Byrne TA, Morrissey TB, Nattakom TV, Ziegler TR, Wilmore DW. Growth hormone, glutamine, and a modified diet enhance nutrient absorption in patients with severe short bowel syndrome. JPEN J Parenter Enteral Nutr. 1995;19:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 177] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Sugiura T, Tashiro T, Yamamori H, Takagi K, Hayashi N, Itabashi T, Toyoda Y, Sano W, Nitta H, Hirano J. Effects of total parenteral nutrition on endotoxin translocation and extent of the stress response in burned rats. Nutrition. 1999;15:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Foitzik T, Kruschewski M, Kroesen AJ, Hotz HG, Eibl G, Buhr HJ. Does glutamine reduce bacterial translocation A study in two animal models with impaired gut barrier. Int J Colorectal Dis. 1999;14:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | O'Dwyer ST, Michie HR, Ziegler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg. 1988;123:1459-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 187] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Welsh FK, Farmery SM, MacLennan K, Sheridan MB, Barclay GR, Guillou PJ, Reynolds JV. Gut barrier function in malnourished patients. Gut. 1998;42:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Lyons A, Kelly JL, Rodrick ML, Mannick JA, Lederer JA. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann Surg. 1997;226:450-48; discussion 450-48;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 169] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Rongione AJ, Kusske AM, Ashley SW, Reber HA, McFadden DW. Interleukin-10 prevents early cytokine release in severe intraabdominal infection and sepsis. J Surg Res. 1997;70:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | McKay DM, Baird AW. Cytokine regulation of epithelial permeability and ion transport. Gut. 1999;44:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | O'Boyle CJ, MacFie J, Dave K, Sagar PS, Poon P, Mitchell CJ. Alterations in intestinal barrier function do not predispose to translocation of enteric bacteria in gastroenterologic patients. Nutrition. 1998;14:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |