Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1302
Revised: June 4, 2003
Accepted: June 11, 2002
Published online: June 15, 2003
AIM: To evaluate the roles and mechanisms of celecoxib in inducing proliferation inhibition and apoptosis of human cholangiocarcinoma cell lines.
METHODS: Cyclooxygenase-2-overexpressing human cholangiocarcinoma cell line QBC939 and cyclooxygenase-2-deficient human cholangiocarcinoma cell line SK-CHA-1 were used in the present study. The anti-proliferative effect was measured by methabenzthiazuron (MTT) assay; apoptosis was determined by transferase-mediated dUTP nick end labeling (TUNEL) detection and transmission electron microscopy (TEM). Cell cycle was analyzed by flow cytometry (FCM). The PGE2 levels in the supernatant of cultured cholangiocarcinoma cells were quantitated by enzyme-linked immunoabsordent assay (ELISA).
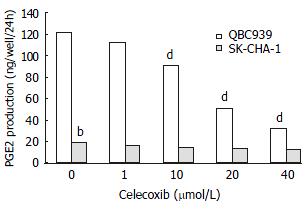
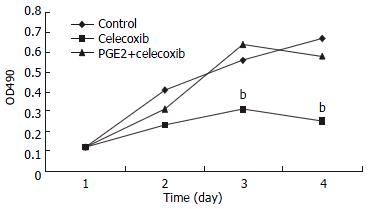
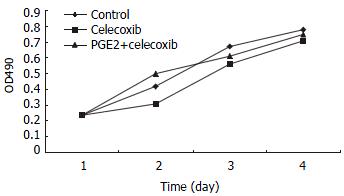
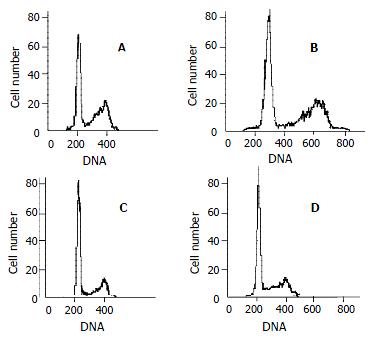
RESULTS: Celecoxib suppressed the production of PGE2 and inhibited the growth of QBC939 cells. Celecoxib at 10, 20, and 40 μmol/L inhibited PGE2 production by 26%, 58%, and 74% in QBC939 cells. The PGE2 level was much lower constitutively in SK-CHA-1 cells (18.6 ± 3.2) compared with that in QBC939 (121.9 ± 5.6) cells (P < 0.01) and celecoxib had no significant influence on PGE2 level in the SK-CHA-1 cells. The PGE2 concentration in SK-CHA-1 cells also reduced but not significantly after treatment with celecoxib. The PGE2 concentration in SK-CHA-1 cells was (16.5 ± 2.9) ng/well, (14.8 ± 3.4) ng/well, (13.2 ± 2.0) ng/well and (12.6 ± 3.1) ng/well respectively, when pre-treated with 1 μmol/L, 10 μmol/L, 20 μmol/L and 40 μmol/L of celecoxib for 48 h (P > 0.05, vs control). The anti-proliferation effect of celecoxib (20 μmol/L) on QBC939 cells was time-dependent, it was noticeable on day 2 (OD490 = 0.23 ± 0.04) and became obvious on day 3 (OD490 = 0.31 ± 0.07) to day 4 (OD490 = 0.25 ± 0.06), and the OD490 in the control group (day 1) was 0.12 ± 0.03 (P < 0.01, vs control). The anti-proliferation effect of celecoxib could be abolished by the addition of 200 pg/mL PGE2. The proliferation of SK-CHA-1 cells was inhibited slightly by celecoxib, the cell density OD490 in the presence of celecoxib and in control group was 0.31 ± 0.04 and 0.42 ± 0.03 respectively on day 2 (P > 0.05), 0.58 ± 0.07 and 0.67 ± 0.09 respectively on day 3 (P > 0.05), and 0.71 ± 0.08 and 0.78 ± 0.06 respectively on day 4 (P > 0.05). Celecoxib induced proliferation inhibition and apoptosis by G1-S cell cycle arrest: the percentage of QBC939 cells in G0-G1phase after treatment with 40 μmol/L (74.6 ± 66.21) and 20 μmol/L (68.63 ± 4.36) celecoxib increased significantly compared with control cells (54.41 ± 5.12, P < 0.01). The percentage of SK-CHA-1 cells in G0-G1 phase after treatment with various concentrations of celecoxib didn't change significantly compared with control cells. The TUNEL index was much higher in QBC939 cells treated with 20 μmol/L celecoxib for 2 d (0.063 ± 0.018) and for 4 d (0.102 ± 0.037) compared with control cells (0.017 ± 0.004, P < 0.01).
CONCLUSION: The current in vitro study indicates that inhibition of proliferation and induction of apoptosis in human cholangiocarcinoma cells by cyclooxygenase-2 specific inhibitor celecoxib may involve in COX-dependent mechanisms and PGE2pathway. Celecoxib as a chemopreventive and chemotherapeutic agent might be effective primarily on COX-2-expressing cholangiocarcinoma.
-
Citation: Wu GS, Zou SQ, Liu ZR, Tang ZH, Wang JH. Celecoxib inhibits proliferation and induces apoptosis
via prostaglandin E2 pathway in human cholangiocarcinoma cell lines. World J Gastroenterol 2003; 9(6): 1302-1306 - URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1302.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1302
Prostaglandins (PGs) are important in the proliferation of various types of cancer cells[1-13]. PGs are synthesized by two isoforms of cyclooxygenase (COX) enzymes, COX-1 and COX-2, each of which displays distinct physiological profile. Inducible isozyme COX-2 has been shown to be important in carcinogenesis[14-26]. PGE2 is the major metabolite of arachidonic acid in many human cells[27,28]. The selective COX-2 inhibitors are currently being evaluated for their effectiveness as chemopreventive and chemotherapeutic agents[29-32]. However, the effects of specific inhibitor of COX-2 on the proliferation of human carcinoma cells remain to be investigated. There are many controversies on whether or not these effects are mediated predominantly through the inhibition of COX-2 activity and prostaglandin synthesis[33]. Our previous studies have demonstrated that overexpression of COX-2 may play a crucial role in the carcinogenesis and development of extra-hepatic cholangiocarcinoma. In this study we aimed to explore the effects and mechanism of celecoxib and the role of PGE2 in inducing proliferation inhibition and apoptosis of COX-2 overexpressing human cholangiocarcinoma cell line QBC939 and COX-2-deficient human cholangiocarcinoma cell line SK-CHA-1.
Human extra-hepatic cholangiocarcinoma cells SK-CHA-1 were a gift from Professor A. Knuth (Frankfurt, Germany)[35]; and human cholangiocarcinoma cell line QBC939 was established by Professor Wang SG in the Third Military Medical University, China, and was offered to us as a gift[34]. Both cells were maintained as mono-layers in Dulbecco's modified Eagle'smedium (DMEM) supplemented with 10% fetal bovine serum (FBS, Gibco. USA.), 100 units/mL penicillin and 100 mg/mL streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. They were subcultivated every 3-5 d and given fresh medium every other day. Cholangiocarcinoma cells at 70%-80% subconfluent were employed in all experiments. PGE2ELISA detection kit was purchased from Jingmei Biotech Co., Wuhan, China. TUNEL kit was purchased from Boster Co., Wuhan, China. PGE2 was purchased from Sigma, USA. Celecoxib was synthesized by Dr. Mei ZN (Wuhan University, China) and given to us as a gift[36]. Stock solution was prepared in dimethylsulfoxide (DMSO) and stored at -20 °C. In all experiments DMSO final concentration in the medium was ≤ 0.1%.
MTT assay The human cholangiocarcinoma cells QBC939 and SK-CHA-1 proliferation status were determined by MTT assay. Cholangiocarcinoma cells were seeded at a density of 1 × 104 cells per well in flat-bottomed 96-well microplates. 12 h after incubation, cells were treated with celecoxib (40, 20, 10, or 0 μmol/L respectively). In some experiments 200 pg/mL PGE2 was added to cells prior to addition of celecoxib. After 1, 2, 3, or 4 d incubation, 20 μL MTT (5 g/L) was added to each well and incubated for 4 h. Supernatant was then removed and 150 μLDMSO was added. It was shaken for 5 min until the crystal was dissolved. OD490nm value was measured by an enzyme-linked immunoabsorbent assay reader. The negative control well had no cells and was used as zero point of absorbance. Each well was read three times in triplicate.
TUNEL Preparation of specimens: cholangiocarcinoma cells QBC939 were subcultured on coverslips in 6-well culture plates. After 12 h, cells were treated with 20 μmol/L celecoxib. Every day medium and celecoxib were changed. After 2 and 4 d the coverslips were taken out and fixed with 4% fresh polyformaldehyde in PBS (pH7.4-7.6) for 30 min at room temperature. Cell apoptosis was measured by TUNEL method according to the instruction of the kit. Cells were washed with PBS for 2 min, 3 times, followed by washing with distilled water for 2 min, 3 times. Cells were soaked in fresh 3% H2O2 for 10 min, and then rinsed with distilled water for 2 min, 3 times. Cells were digested with proteinase K (diluted 1:100 by TBS) for 5 min at 37 °C, and were rinsed with distilled water for 2 min, 3 times. Labelling buffer (20 μL/sample) was added to keep the slides wet. TDT and DIG-d-UTP (1 μL each) were mixed in 18 μL labelling buffer. The redundant liquid was removed and labelling reagent (20 μL/sample) was added. The slides were put in a humidified box and incubated for 2 h at 37 °C. The slides were washed with TBS for 2 min, 3 times. Blocking solution (50 μL/sample) was added to the slides for 30 min at room temperature. The blocking solution was removed from the slides. The biotin-DIG antibody was diluted with a blocking solution at a ratio of 1:100, and 50 μL/sample of it was added to the slides. The slides were kept in a humidified box, incubated at 37°C for 30 min, and followed by washing with TBS for 5 min, 3 times. SABC was diluted to 1:100 with TBS and added to the slides. They were incubated at 37 °C for 30 min and washed with TBS for 5 min, 3 times. BCIP/NBT was diluted to 1:20 with TBS, and added to the slides. They were incubated at 37 °C for 10-30 min. The reaction was monitored under microscope: when purplish red was developed, the slides were washed with distilled water. After being stained with nuclear fast red, the slides were sealed with glycerite. Substitution of PBS for TUNEL staining solution was used as negative control. Three hundred cells were counted, and the TUNEL index was expressed as the number of positive cells/the total number of cells.
ELISA The PGE2 levels in the supernatant of cultured human cholangiocarcinoma cells QBC939 and SK-CHA-1 were quantitated by ELISA. Cells were seeded into 4.0 × 105/well microplates and allowed to adhere overnight. The cells were then incubated in the presence or absence of celecoxib for 24 h. The supernatants were aspirated and centrifuged to prepare for the detection of PGE2. Supernatant (0.5 mL) was added into 1 N HCl (0.1 mL) and centrifuged for 10 min at room temperature, then 1.2 N NaOH (0.1 mL) was used to neutralize the acidified samples. Standard solution (200 μL per well) or activated samples were added into the microplates. Then the steps for ELISA were performed as instructed. The value of OD of each well was measured at 450nm. The supernatants were harvested in triplicates and the experiment was performed twice.
FCM Human cholangiocarcinoma cells QBC939 and SK-CHA-1 were trypsinized and plated in 6-well culture dishes in the presence of celecoxib (40, 20, 10, 0 μmol/L respectively). After 48 h, cells were harvested, centrifuged at low speed and fixed in 70% ethanol. After overnight incubation at 4 °C, cells were stained with 50 μg/mL propidium iodide in the presence of RNAse A (10 μg/ml) and 0.1% Triton X-100 and measured with a flow cytometer. The experiments were repeated three times.
TEM After treatment with celecoxib for 3 d cholangiocarcinoma cells QBC939 were digested by 0.25% trypsin and collected. Cells were rinsed with PBS and fixed with 3% glutaraldehyde for 30 min. After routine embedding and sectioning, cells were examined under electron microscope.
Data were expressed as mean ± standard deviation. Student's t-test was used for statistical analysis. P < 0.05 indicates significant difference.
The concentration of PGE2 in culture medium of each cell line treated with or without celecoxib is shown in Figure 1. Celecoxibat 10 μmol/L inhibited PGE2 production in QBC939 cells by 26%. With 20 μmol/L and 40 μmol/L of celecoxib, PGE2production was further inhibited by 58% and 74%, which were statistically significant (P < 0.01, vs control). The PGE2 level was much lower constitutively in SK-CHA-1 cells (18.6 ± 3.2) compared with that in QBC939 (121.9 ± 5.6) cells (t test, P < 0.01). The PGE2 concentration in SK-CHA-1 cells was also reduced, but not significantly after treatment with celecoxib. The PGE2 concentration in SK-CHA-1 cells was (16.5 ± 2.9) ng/well, (14.8 ± 3.4) ng/well, (13.2 ± 2.0) ng/well and (12.6 ± 3.1) ng/well respectively, when pre-treated with 1 μmol/L, 10 μmol/L, 20 μmol/L and 40 μmol/L of celecoxib for 48 h (P > 0.05, vs control).
QBC939 and SK-CHA-1 cells were incubated in the presence or absence of celecoxib (20 μmol/L) and the cell density OD490 was measured. As shown in Figure 2, proliferation inhibition of QBC939 by celecoxib was time-dependent: it was noticeable on day 2 (OD490 = 0.23 ± 0.04) and became obvious on day 3 (OD490 = 0.31 ± 0.07) to day 4 (OD490 = 0.25 ± 0.06), and the OD490 in the control group (day 1) was 0.12 ± 0.03 (P < 0.01, vs control). The proliferation of SK-CHA-1 cells was inhibited slightly by celecoxib, but the effect was not statistically significant (Figure 3). The cell density OD490 in the presence of celecoxib and in control group was 0.31 ± 0.04 and 0.42 ± 0.03 respectively on day 2 (P > 0.05), 0.58 ± 0.07 and 0.67 ± 0.09 respectively on day 3 (P > 0.05), and 0.71 ± 0.08 and 0.78 ± 0.06 respectively on day 4 (P > 0.05).
To investigate whether the anti-proliferation effect of celecoxib on QBC939 cells was due to suppression of PGE2 production by QBC939 cells, 200 pg/mL PGE2 was added to QBC939 cells prior to the addition of celecoxib, and MTT assay was performed (Figure 2). The anti-proliferation effect of celecoxib on QBC939 cells was abolished by PGE2. However, addition of 200 pg/mL PGE2 had no significant influence on the proliferation of SK-CHA-1 cells when pre-treated with 20 μmol/L celecoxib (Figure 3).
The TUNEL index of QBC939 cells treated with 20 μmol/L celecoxib for 2 d (0.063 ± 0.018) and 4 d (0.102 ± 0.037) was much higher compared with control cells (0.017 ± 0.004, P < 0.01).
Cell cycle analysis by flow cytometry showed that the percentage of QBC939 cells in G0-G1 phase after treatment with 40mmol/L (74.66 ± 6.21) and 20 μmol/L (68.63 ± 4.36) increased significantly compared with control cells (54.41 ± 5.12, P < 0.01, Figure 4). The percentage of SK-CHA-1 cells in G0-G1 phase after treatment with various concentrations of celecoxib did not change significantly compared with control cells.
QBC939 cells were treated for 3 d with 20 μmol/L of celecoxib. The chromatin became condensed and attached to the inner surface of nuclear membrane.
A substantial body of evidence indicates that COX and PGs are important in carcinogenesis. COX catalyzes the synthesis of PGs from arachidonic acid. Several PGs, most notably PGE2, can promote tumorigenesis by stimulating angiogenesis, inhibiting immune surveillance[37-40], modulating several signal transduction pathways[41-44]. Several studies have demonstrated that COX-2 selective inhibitor celecoxib has significant efficacy in animal cancer models: celecoxib inhibited intestinal tumor multiplicity by up to 71% compared with controls in the Min mouse model and inhibited colorectal tumor burden in the rat azoxymethane (AOM) model[45-48]. Recently celecoxib has been approved by FDA to reduce the number of adenomatous colorectal polyps in patients with familial adenomatous polyposis (FAP). However, the exact mechanisms that account for the anti-proliferative effects of celecoxib are still not fully understood. It is still controversial that whether or not these effects are mediated predominantly through the inhibition of COX-2 activity and prostaglandin synthesis. Several studies have shown both COX-dependent and COX-independent mechanisms are involved in non-steroidal anti-inflammatory drug (NSAIDs) induced growth in human colorectal tumor cells[49].
Our previous studies have demonstrated that overexpression of COX-2 may play a crucial role in the carcinogenesis and development of extra-hepatic cholangiocarcinoma. In the present study we found the PGE2 level was much lower constitutively in COX-2-deficient human cholangiocarcinoma cell line SK-CHA-1 cells than that in COX-2 overexpressing human cholangiocarcinoma cell line QBC939. In this study we have shown that the proliferation of QBC939 cells was inhibited by celecoxib in a time- and dose-dependent manner. Our study also showed celecoxib had no significant influence on the SK-CHA-1 cells. These findings indicate that COX-2 inhibitor might be an effective anti-proliferative agent, especially against cancer cells that express COX-2 and produce high-level PGE2. Our data demonstrated that celecoxib suppressed the production of PGE2 in QBC939 cells, and the anti-proliferative effect of celecoxib could be abolished by addition of PGE2. These results suggest that COX-2 might play a central role in production of PGE2 and the specific inhibition of COX-2 inhibits proliferation and induces apoptosis of QBC939 cells via suppression of PGE2 production. Our data also indicate that celecoxib inhibits proliferation and induces apoptosis of human cholangiocarcinoma QBC939 cells by an accumulation of cells in the G0/G1 phase and the inhibition of G0/G1 phase transition to S phase.
In summary, our results in the present study demonstrate that inhibition of proliferation and induction of apoptosis by celecoxib in human cholangiocarcinoma cells may involve in COX-dependent mechanisms and PGE2 pathway and these findings also suggest that celecoxib, as a chemopreventive and chemotherapeutic agent may be effective primarily on COX-2-expressing cholangiocarcinoma.
Edited by Zhu L and Bo XN
| 1. | Boström PJ, Aaltonen V, Söderström KO, Uotila P, Laato M. Expression of cyclooxygenase-1 and -2 in urinary bladder carcinomas in vivo and in vitro and prostaglandin E2 synthesis in cultured bladder cancer cells. Pathology. 2001;33:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Fan XM, Wong BC, Lin MC, Cho CH, Wang WP, Kung HF, Lam SK. Interleukin-1beta induces cyclo-oxygenase-2 expression in gastric cancer cells by the p38 and p44/42 mitogen-activated protein kinase signaling pathways. J Gastroenterol Hepatol. 2001;16:1098-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Wu GS, Zou SQ, Luo XW, Wu JH, Liu ZR. Proliferative activity of bile from congenital choledochal cyst patients. World J Gastroenterol. 2003;9:184-187. [PubMed] |
| 4. | Chasseing NA, Hofer E, Bordenave RH, Shanley C, Rumi LS. Bone marrow fibroblasts in patients with advanced lung cancer. Braz J Med Biol Res. 2001;34:1457-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Berger S, Siegert A, Denkert C, Köbel M, Hauptmann S. Interleukin-10 in serous ovarian carcinoma cell lines. Cancer Immunol Immunother. 2001;50:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Venza I, Giordano L, Piraino G, Medici N, Ceci G, Teti D. Prostaglandin E2 signalling pathway in human T lymphocytes from healthy and conjunctiva basal cell carcinoma-bearing subjects. Immunol Cell Biol. 2001;79:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Chen X, Yang CS. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis. 2001;22:1119-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | McHowat J, Creer MH, Rickard A. Stimulation of protease activated receptors on RT4 cells mediates arachidonic acid release via Ca2+ independent phospholipase A2. J Urol. 2001;165:2063-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Krishnan K, Ruffin MT, Normolle D, Shureiqi I, Burney K, Bailey J, Peters-Golden M, Rock CL, Boland CR, Brenner DE. Colonic mucosal prostaglandin E2 and cyclooxygenase expression before and after low aspirin doses in subjects at high risk or at normal risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:447-453. [PubMed] |
| 10. | Lim JW, Kim H, Kim KH. Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab Invest. 2001;81:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Akhtar M, Cheng Y, Magno RM, Ashktorab H, Smoot DT, Meltzer SJ, Wilson KT. Promoter methylation regulates Helicobacter pylori-stimulated cyclooxygenase-2 expression in gastric epithelial cells. Cancer Res. 2001;61:2399-2403. [PubMed] |
| 12. | Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075-18081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 486] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 13. | Faas FH, Dang AQ, White J, Schaefer R, Johnson D. Increased prostatic lysophosphatidylcholine acyltransferase activity in human prostate cancer: a marker for malignancy. J Urol. 2001;165:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483-487. [PubMed] |
| 15. | Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796-800. [PubMed] |
| 16. | Cheng J, Imanishi H, Iijima H, Shimomura S, Yamamoto T, Amuro Y, Kubota A, Hada T. Expression of cyclooxygenase 2 and cytosolic phospholipase A(2) in the liver tissue of patients with chronic hepatitis and liver cirrhosis. Hepatol Res. 2002;23:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Davies G, Martin LA, Sacks N, Dowsett M. Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a possible role for COX-2 inhibitors in breast cancer chemoprevention. Ann Oncol. 2002;13:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM. Cyclooxygenase 2- and prostaglandin E(2) receptor EP(2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res. 2002;62:506-511. [PubMed] |
| 19. | Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198-204. [PubMed] |
| 20. | Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Ristimäki A, Nieminen O, Saukkonen K, Hotakainen K, Nordling S, Haglund C. Expression of cyclooxygenase-2 in human transitional cell carcinoma of the urinary bladder. Am J Pathol. 2001;158:849-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Kulkarni S, Rader JS, Zhang F, Liapis H, Koki AT, Masferrer JL, Subbaramaiah K, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin Cancer Res. 2001;7:429-434. [PubMed] |
| 24. | Tong BJ, Tan J, Tajeda L, Das SK, Chapman JA, DuBois RN, Dey SK. Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-delta in human endometrial adenocarcinoma. Neoplasia. 2000;2:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol. 2001;76:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 26. | Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, Kosuge T, Yoshida S. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001;91:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Kakiuchi Y, Tsuji S, Tsujii M, Murata H, Kawai N, Yasumaru M, Kimura A, Komori M, Irie T, Miyoshi E. Cyclooxygenase-2 activity altered the cell-surface carbohydrate antigens on colon cancer cells and enhanced liver metastasis. Cancer Res. 2002;62:1567-1572. [PubMed] |
| 28. | Sumitani K, Kamijo R, Toyoshima T, Nakanishi Y, Takizawa K, Hatori M, Nagumo M. Specific inhibition of cyclooxygenase-2 results in inhibition of proliferation of oral cancer cell lines via suppression of prostaglandin E2 production. J Oral Pathol Med. 2001;30:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Hosomi Y, Yokose T, Hirose Y, Nakajima R, Nagai K, Nishiwaki Y, Ochiai A. Increased cyclooxygenase 2 (COX-2) expression occurs frequently in precursor lesions of human adenocarcinoma of the lung. Lung Cancer. 2000;30:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Tsubouchi Y, Mukai S, Kawahito Y, Yamada R, Kohno M, Inoue K, Sano H. Meloxicam inhibits the growth of non-small cell lung cancer. Anticancer Res. 2000;20:2867-2872. [PubMed] |
| 31. | Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60:5767-5772. [PubMed] |
| 32. | Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, Kelloff GJ, Hill DL, Seibert K. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60:5599-5602. [PubMed] |
| 33. | Grösch S, Tegeder I, Niederberger E, Bräutigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742-2744. [PubMed] |
| 34. | Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, Prellwitz W, Meyer zum Büschenfelde KH. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 176] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Wang SG, Han BL, Duan HC, Chen YS, Peng ZM. Establishment of the extrahepatic cholangiocarcinoma cell line. Zhonghua Shiyan Waike Zazhi. 1997;14:67-68. |
| 36. | Mei ZY, Shi Z, Wang XH, Luo XD. Synthesis of COX-2 Inhibitor Celecoxib. Zhongguo Yiyao Gongye Zazhi. 2000;31:433-434. |
| 37. | Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 38. | Vanaja DK, Grossmann ME, Celis E, Young CY. Tumor prevention and antitumor immunity with heat shock protein 70 induced by 15-deoxy-delta12, 14-prostaglandin J2 in transgenic adenocarcinoma of mouse prostate cells. Cancer Res. 2000;60:4714-4718. [PubMed] |
| 39. | O'Byrne KJ, Dalgleish AG, Browning MJ, Steward WP, Harris AL. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur J Cancer. 2000;36:151-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Morecki S, Yacovlev E, Gelfand Y, Trembovler V, Shohami E, Slavin S. Induction of antitumor immunity by indomethacin. Cancer Immunol Immunother. 2000;48:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Jang BC, Sanchez T, Schaefers HJ, Trifan OC, Liu CH, Creminon C, Huang CK, Hla T. Serum withdrawal-induced post-transcriptional stabilization of cyclooxygenase-2 mRNA in MDA-MB-231 mammary carcinoma cells requires the activity of the p38 stress-activated protein kinase. J Biol Chem. 2000;275:39507-39515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Paik JH, Ju JH, Lee JY, Boudreau MD, Hwang DH. Two opposing effects of non-steroidal anti-inflammatory drugs on the expression of the inducible cyclooxygenase. Mediation through different signaling pathways. J Biol Chem. 2000;275:28173-28179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res. 2000;60:2399-2404. [PubMed] |
| 44. | Higashi Y, Kanekura T, Kanzaki T. Enhanced expression of cyclooxygenase (COX)-2 in human skin epidermal cancer cells: evidence for growth suppression by inhibiting COX-2 expression. Int J Cancer. 2000;86:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 45. | Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Jacoby RF, Cole CE, Tutsch K, Newton MA, Kelloff G, Hawk ET, Lubet RA. Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res. 2000;60:1864-1870. [PubMed] |
| 47. | Reddy BS, Rao CV, Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res. 1996;56:4566-4569. [PubMed] |
| 48. | Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409-412. [PubMed] |
| 49. | Richter M, Weiss M, Weinberger I, Fürstenberger G, Marian B. Growth inhibition and induction of apoptosis in colorectal tumor cells by cyclooxygenase inhibitors. Carcinogenesis. 2001;22:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |