INTRODUCTION
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that play an essential role in the activation of lymphocytes[1-10]. Among APCs, which also include macrophages and B cells, only DCs are believed to be capable of activating naive T cells. DC function is regulated by their state of maturation. Immature DCs resident in nonlymphoid tissues such as normal liver are deficient at antigen capture and progressing processing[11,12]. "Maturation" of DC can be induced by microbial stimuli, proinflammatory cytokines, as well as through interaction with CD40L-CD40 cross-linking[13-20]. The control of DC maturation and activation plays an important role in regulating their T cell priming functions[21]. Significantly, engagement of CD40 expressed on DCs with CD40L expressed on T cells not only stimulates maturation and cytokine production but also enhances DC survival and activation[19,22,23]. Mature DCs are highly immunogenic due to high levels of expression of MHC I and II, costimulatory and adhesion molecules, including B7-1, B7-2, CD40, and ICAM-1[11-19]. In the case of experimental skin, heart, or kidney allografts, mature dendritic cells resident in donor tissue have been implicated as the principal instigators of rejection. Whereas, DCs derived from normal liver display an immature phenotype with absence of costimulatory molecules (CD40, CD80 and CD86) surface expression, low levels of MHC class I and II, and as a consequence, low stimulatory capacity for naive allogeneic T cells. Unlike mature DC, these liver-derived immature DCs do not induce detectable levels of intracytoplasmic IFN-γ in allogeneic CD4+ cells in 72-h MLR, and elicite very low levels of CTLs in vitro[11,12]. It has been observed that liver-derived[11,24] or bone marrow-derived immature DCs[25], propagated in vitro and lacking surface costimulatory molecules, can prolong heart or pancreatic islet allograft survival. Whereas, marked augmentation of DCs numbers and maturation of DCs in liver allografts by donor treatment with the hematopoietic growth factor fms-like tyrosine kinase 3 (Flt3) ligand (FL) results in acute liver graft rejection[26,27].
Although the significance of DCs as regulators of transplantation immunity is beyond doubt, little is known about intracellular mechanisms specifically responsible for regulation of DC activation and maturation. Previous studies have suggested that NF-κB may play a key role in DC maturation[20,28-30], and NF-κB inhibition could impair the maturation and function of DC[28,29].
A20 is a zinc finger protein originally identified as a TNF- inducible gene product in endothelial cells (EC), and has been shown to be dependent upon NF-κB for its expression. A20 is expressed in a variety of cell types including fibroblasts, B, T, and β-cells in response to different stimuli including LPS, IL-1 and CD40 cross-linking[31-36]. A20 is itself a NF-κB- dependent gene and is part of a negative regulatory loop critical for modulation of cell activation[37,38]. A20 serves a broad cytoprotective function in EC by protecting EC from apoptosis and down-regulating inflammatory responses via NF-κB inhibition[39]. A20-/- knock-out mice are born cachectic and die within 3 wk from severe and uncontrolled inflammation that further confirms the potent anti-inflammatory function of A20[40]. A20 is also part of the physiologic NF-κB-dependent survival response of hepatocytes to injury, limited expression of A20 in hepatocytes drastically improves the fate of mice in the D-gal/LPS model of toxic FHF where A20 protects hepatocytes from apoptosis and promotes the liver regeneration[37]. In addition, investigation has shown that A20 expression is up-regulated in human renal allografts in response to immune injury inferred by acute rejection, and the result suggests that A20 could limit graft injury[41].
Although A20 is a very effective inhibitor of NF-κB activation induced by LPS, IL-1 and CD40 cross-linking, little is known about the role of A20 in the regulation of maturation of DCs derived from allogeneic liver grafts accompanied by acute rejection.
The purpose of the present study was to investigate the binding activity of NF-κB DNA and A20 expression in mature DCs derived from allogeneic partial liver grafts undergoing acute rejection in rats. Attempts were made also to correlate A20 expression in DCs derived from liver grafts with the acceptance of allogeneic liver grafts.
MATERIALS AND METHODS
Animals
Sixty donor male SD rats and sixty recipient male LEW rats weighing 220-300 g were randomly divided into whole liver transplantation group and partial liver transplantation group. Allogeneic whole and 50% partial liver transplantation were performed using a SD to LEW combination. The animals were purchased from Chinese Academy of Sciences and Sichuan University. They were maintained with a 12-hour light/dark cycle in a conventional animal facility with water and commercial chow provided ad libitum, with no fasting before the transplantation.
Liver transplantation
All operations were performed under ether anesthesia in clean but not sterile conditions. All surgical procedures were performed from 8 a.m to 5 p.m . Donors and recipients of similar weight (± 10 g) were chosen. Liver reduction was achieved by removing the left lateral lobe and the two caudate lobes, which resulted in a 50% reduction of the liver mass. Whole liver transplantation (WLT) and partial liver transplantation (PLT) were performed according to the method described in our previous study[42].
Histology
Part of liver tissues was sectioned and preserved in 10% formalin, embedded in paraffin, cut with microtome, and stained with hematoxylin and eosin. The histological grading of rejection was determined according to the criteria described by Williams.
Propagation and purification of liver graft-derived DC populations
DCs from liver graft 0 hour and 4 d after the transplantation were propagated in GM-CSF from nonparenchymal cells (NPC) isolated from collagenase-digested liver graft tissue, as described by Lu et al[24]. Nonadherent cells, released spontaneously from proliferating cell clusters, were collected after culture for 10 d, and purified by centrifugation 500 × g, for 10 min at room temperature on a 16% w/v metrizamide gradient (DC purity 80%-85%).
Morphological and phenotypical features of DCs
Morphological characteristics of DCs derived from liver graft were observed by electron microscopy. Expression of cell surface molecules was quantitated by flow cytometry as described in our previous study[42]. Aliquots of 2 × 105 DCs propagated for 10 d in vitro were incubated with the following primary mouse anti - rat mAbs against OX62, CD40 (Serotec, USA), or rat IgG as an isotype control for 60 min on ice (1 μg/mL diluted in PBS/1.0% FCS). The cells were washed with PBS/1.0% FCS and labeled with FITC-conjugated goat anti-mouse IgG, diluted 1/50 in PBS/1.0% FCS for 30 min on ice. At the end of this incubation, cells were washed, propidium iodide/PBS were added, and the cells were subsequently analyzed in an FACS-4200 flow cytometer (Becton-Dikison, USA).
Isolation of nuclear proteins
Nuclear proteins were isolated from DCs extract by placing the sample in 0.9 mL of ice-cold hypotonic buffer [10 mM•l-1HEPES (pH7.9), 10 mM•l-1 KCl, 0.1 mM•l-1 EDTA, 0.1 mM•l-1 ethylene glycol tetraacetic acid, 1 mM•l-1 DTT; Protease inhibitors (aprotinin, pepstatin, and leupeptin, 10 mg•l-1 each)]. The homogenates were incubated on ice for 20 min, vortexed for 20 s after adding 50 μL of 10% Nonide-P40, and then centrifuged for 1 min at 4 °C in an Eppendorf centrifuge. Supernatants were decanted, the nuclear pellets after a single wash with hypotonic buffer without Nonide-P40 were suspended in an ice-cold hypertonic buffer [20 mM•l-1 HEPES(pH7.9), 0.4 M•l-1 NaCl, 1 mM•l-1 EDTA, 1 mM•l-1 DTT; Protease inhibitors], incubated on ice for 30 min at 4 °C, mixed frequently, and centrifuged for 15 min at 4 °C. The supernatants were collected as nuclear extracts and stored at -70 °C. Concentrations of total proteins in the samples were determined according to the method of Bradford.
Electrophoretic mobility shift assay (EMSA) for NF-κB activation of DCs
NF-κB binding activity was performed in a 10-μL binding reaction mixture containing 1 × binding buffer [50 mg•l-1 of double-stranded poly (dI-dC), 10 mM•l-1 Tris-HCl (pH7.5), 50 mM•l-1 NaCl, 0.5 mM•l-1 EDTA, 0.5 mM•l-1DTT, 1 mM•l-1 MgCl2, and 100 mL•l-1 glycerol], 5 mg of nuclear protein, and 35 fmol of double-stranded NF-κB consensus oligonucleotide (5'-AGTTGAGGGGACTTTCCCAGG-3') that was endly labeled with γ-32P (111TBq mM-1 at 370 GBq-1) using T4 polynucleotide kinase. The binding reaction mixture was incubated at room temperature for 20 min and analyzed by electrophoresis on 7% nondenaturing polyacrylamide gels. After electrophoresis, the gels were dried by Gel-Drier (Biol-Rad Laboratories, Hercules, CA) and exposed to Kodak X-ray films at -70 °C.
Western blotting for IL-12 p70 and zinc finger protein A20 expression in DCs
DCs cultured for 10 d in vitro were starved in serum-free medium for 4 h at 37 °C. These cells were washed twice in cold PBS, resuspended in 100 μL lysis buffer (1% Nonidet -P40, 20 mM Tris-HCl, pH8.0, 137 mM NaCl, 10% glycerol, 2 mM EDTA, 10 μg•L-1 leupeptin, 10 μg•L-1 aprotinin, 1mM PMSF, and 1 mM sodium orthovanadate), and total cell lysates were obtained. The homogenates were centrifuged at 10000 × g for 10 min at 4 °C. Cell lysates (20 μg) were electrophoresed on SDS-PAGE gels, and transferred to PVDC membranes for Western blot analysis. Briefly, PVDC membranes were incubated in a blocking buffer for 1 hour at room temperature, then incubated for 2 h with Abs raised against IL-12 p70 and A20 (Santa Cruz, CA). The membranes were washed and incubated for 1 hour with HRP-labeled IgG. Immunoreactive bands were visualized by ECL detection reagent. The binding bands were quantified by scanning densitometer of a Bio-Image Analysis System. The results were expressed as relative optical density.
Statistics analysis
Statistic analysis of data was performed using the Student's t-test; P < 0.05 was considered statistically significant.
RESULTS
Histological rejection
Histological rejection features of allografted livers were compared between the whole and partial groups on day 4 after the transplantation, allogeneic whole liver grafts demonstrated no rejection. In contrast, partial liver grafts demonstrated moderate to severe rejection, including inflammatory cellular infiltration in the portal tract, endotheliitis, bile duct damage and hepatocytes necrosis.
Phenotypic characteristics of liver graft-derived DCs propagated in vitro
As shown in our previous study[42], after cultured for 10 d in the presence of GM-CSF, DCs both from whole and partial liver grafts displayed typical morphological features of DC, including anomalous shape, bigger body, and numerous longer dendrites. Flow cytometry showed 80%-85% of these DCs strongly expressed rat DC - specific OX62 antigen molecule, which suggested that high purity DCs were obtained. Flow cytometric analysis showed that DCs from whole liver grafts and from partial liver grafts 0 hour after the transplantation were negative for the costimulatory molecule CD40 expression, which was an immature phenotype (CD40-), whereas DCs from partial liver graft 4 d after the transplantation showed high level of CD40 expression, which was a mature phenotype(CD40+). These results suggested maturation of DCs resident in allogeneic partial liver graft undergoing acute rejection.
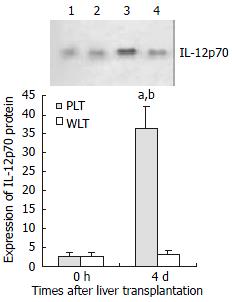
IL-12 p70 protein expression in DCs derived from allogeneic partial liver grafts
Our previous study showed that IL-12 p35 and IL-12 p40 subunit expressions were significantly up-regulated in mature DCs derived from allogeneic partial liver grafts undergoing acute rejection[42]. In the present study, we evaluated IL-12 p70 expression in DCs from allogeneic liver grafts. As shown in Figure 1, DCs derived from both whole and partial liver grafts 0 hour after the transplantation expressed detectable but very low level of IL-12 p70, and expression level of IL-12 p70 in DCs from whole liver graft 4 d after transplantation was not elevated compared with those of DCs from whole liver graft 0 hour after the transplantation (P > 0.05). However, expression of IL-12 p70 in DCs from partial liver graft 4 d after transplantation was markedly increased, and their expression levels were significantly higher than those of DCs both from partial liver graft 0 hour and whole liver graft 4 d after transplantation (P < 0.001).
Figure 1 Expression of IL-12 p70 in liver graft - derived DCs by Western blotting.
Lanes 1, 2: Expression of IL-12 p70 in DCs from partial liver graft and whole liver graft 0 hour after transplantation. Lanes 3, 4: Expression of IL-12 p70 in DCs from partial liver graft and whole liver graft 4 d after transplantation. aP < 0.001 vs 4d WLT group; bP < 0.001 vs 0 hour PLT group.
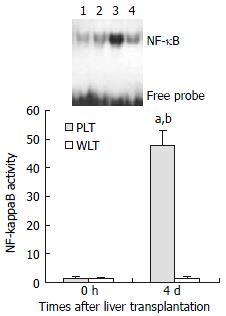
Electrophoretic mobility shift assay (EMSA) for NF-κB activation of DCs
As shown in Figure 2, EMSA analysis showed detectable but very low level of NF-κB activity of DCs derived from both whole and partial liver grafts 0 hour after transplantation, and NF-κB activity of DCs from whole liver graft 4 d after transplantation was not increased compared with those of DCs from liver graft 0 hour after transplantation (P > 0.05). However, NF-κB activity of DCs from partial liver graft 4 d after transplantation was significantly elevated compared with those of DCs from partial liver graft 0 hour and whole liver graft 4 d after transplantation (P < 0.001).
Figure 2 NF-κB activation of DCs derived from allogeneic liver grafts.
Lanes 1, 2: NF-κB activation of DCs from partial liver graft and whole liver graft 0 hour after transplantation. Lanes 3, 4: NF-κB activation of DCs from partial liver graft and whole liver graft 4 d after transplantation. aP < 0.001 vs 4 d WLT group; bP < 0.001 vs 0 hour PLT group.
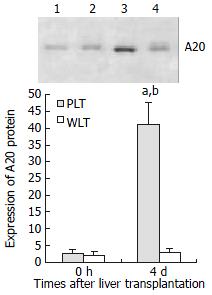
A20 protein expression in DCs derived from partial liver allografts
In order to investigate the intracellular mechanisms specifically responsible for regulation of DC activation and maturation, we evaluated NF-κB inhibitor zinc finger protein A20 expression in DCs derived from allogeneic liver grafts. As shown in Figure 3, DCs from both whole and partial liver grafts 0 hour after the transplantation expressed detectable but very low level of A20, and expression level of A20 in DCs derived from whole liver graft 4 d after transplantation was not increased compared with those of DCs from whole liver graft 0 hour after transplantation (P > 0.05). However, expression of A20 in DCs from partial liver graft 4 d after transplantation was markedly up-regulated, and its expression level was significantly higher than those of DCs both from partial liver graft 0 hour and whole liver graft 4 d after transplantation (P < 0.001).
Figure 3 Expression of A20 protein in DCs derived from allogeneic liver grafts.
Lanes 1, 2: Expression of A20 protein in DCs from partial liver graft and whole liver graft 0 hour after transplantation. Lanes 3, 4: Expression of A20 protein in DCs from partial liver graft and whole liver graft 4days after transplantation. aP < 0.001 vs 4 d WLT group; bP < 0.001 vs 0 hour PLT group.
DISCUSSION
Despite the emergence of DCs as key cellular players in the immune system, the signal transduction events that regulate DC maturation and function have been poorly understood. This study was conducted to explore whether NF-κB activation and its inducible expression gene of A20 could be detected in mature DCs derived from liver allograft undergoing acute rejection. Our aim was to determine whether A20 gene is involved in NF-κB inhibition of these mature DCs. In the present study, it has been shown that engagement of CD40 on DCs derived from allogeneic partial liver grafts undergoing acute rejection leads to a powerful NF-κB activation of these mature DCs, and as a consequence, leads to high level expression of the protective gene A20 in these DCs. Expression of this gene in mature DCs derived from liver graft undergoing acute rejection is consistent with their known potential to be induced in response to NF-κB activation.
It has been shown that DCs derived from allogeneic partial liver grafts undergoing acute rejection displayed mature phenotypic high level CD40 expression and NF-κB activation. Although resting DCs residing in normal liver tissues display only low levels of CD40, B7, and MHC class II molecule expression[11,12,24]. The ischemia/reperfusion injury which is consecutive to the transplantation procedure will rapidly activate them. In addition, partial hepatectomy has been reported to induce the expression of MHC II on Kupffer cell. Interstitial dendritic cells and sinusoidal endothelium in rats, together with the up-regulated TNF-α production after partial hepatectomy would induce the expression of B7 and CD40 molecules on DCs[16]. These factors could stimulate maturation of DCs derived from partial liver grafts. These mature DCs may contribute to the allogeneic liver graft rejection induction. Previously other studies showed that mature DC could provide signals able to trigger T cell proliferation after TCR engagement[43,44]. These accessory, or "costimulatory" signals, are consecutive to interactions between costimulatory molecules present on activated DC such as B7, CD40, and OX40-ligand and their respective counter-receptors, CD28, CD40-ligand, and OX40, on T cell membranes. Several intracellular signals follow the engagement of costimulatory molecules. Interactions of CD40L, CD28, and OX40 with their ligands on DCs activate the transcription factor NF-κB in both T cells and DCs[29,30]. In turn, NF-κB initiates the transcription of numerous genes involved in immune activation, such as chemokines and cytokines[20,30], and also of costimulatory molecules themselves. For instance, CD40 ligation on the APC will up-regulate its expression of B7 molecules. This initiates positive feedback loops ultimately contributing to T cell expansion[45]. Among the costimulatory molecules, CD40 and B7 seem to play a crucial role in alloreactive responses. Indeed, blockade of both CD40 and B7 molecules at the time of transplantation prevents allograft rejection and induces alloreactive T cell anergy[46,47]. In the present study, DCs derived from allogeneic partial liver grafts undergoing acute rejection demonstrated high level expression of CD40, which could interact with the CD40-ligand on T cells, leading to a powerful NF-κB activation and high level of IL-12p70 expression in these mature DCs. Given an essential role for NF-κB transcription in LPS- and CD40L - induced expression of IL-12 (IL-12 p35, p40 and p70) in DCs[20], IL-12 is a key inducer of liver graft rejection[26], together with high level of IL-12 p35 and IL-12 p40 protein expression in the mature DCs derived from allogeneic liver grafts undergoing acute rejection[42]. Our results suggest that NF-κB may play a key role in the maturation of DCs derived from allogeneic liver grafts undergoing acute rejection.
To provide further insights into potential intracellular mechanisms responsible for maturation regulation of DCs derived from allogeneic liver grafts undergoing acute rejection, we measured the protective A20 gene expression in these DCs. Zinc finger protein A20 is a potent inhibitor of NF-κB[31], and although other studies have shown A20 is mainly expressed in endothelial and infiltrating mononuclear cells of human renal allografts undergoing acute rejection[41], little is known about whether it is also involved in the regulation of DC maturation and activation. Our results first demonstrate that high level A20 expression is detected in the mature DCs (which presente significant NF-κB activation) derived from acute rejecting liver allografts, but few A20 is detected in the immature DCs (which present few NF-κB activation) derived from nonrejecting liver allografts. Given A20 is itself a NF-κB - dependent gene and is part of a negative regulatory loop critical for modulation of cell activation[37,38], together with NF-κB which may play a key role in the maturation of DCs derived from liver allografts undergoing acute rejection, it is suggested that A20 expression in these mature DCs derived from liver allografts undergoing acute rejection survives to inhibit NF-κB activation and to limit maturation of these DCs, and as a consequence to limit the graft rejection.
In summary, we demonstrate for the first time an association between NF-κB activation and expression of the protective gene A20 and maturation of DCs derived from liver allografts undergoing acute rejection. NF-κB binding activity and A20 expression in these mature DCs are strongly up-regulated in response to acute rejection. NF-κB may play a key role in the maturation of DCs derived from allogeneic liver grafts undergoing acute rejection, and A20 expression in these mature DCs derived from liver allografts undergoing acute rejection survives to inhibit NF-κB activation and to limit maturation of these DCs.