Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1220
Revised: January 4, 2003
Accepted: January 28, 2003
Published online: June 15, 2003
AIM: To investigate whether troglitazone (TGZ), the peroxisome proliferator-activated receptor (PPAR) gamma ligand, can induce apoptosis and inhibit cell proliferation in human liver cancer cell line HepG2 and to explore the molecular mechanisms.
METHODS: [3-(4, 5)-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT), [3H] Thymidine incorporation, Hochest33258 staining, DNA ladder, enzyme-linked immunosorbent assay (ELISA), RT-PCR, Northern and Western blotting analyses were employed to investigate the effect of TGZ on HepG2 cells and related molecular mechanisms.
RESULTS: TGZ was found to inhibit the growth of HepG2 cells and to induce apoptosis. During the process, the expression of COX-2 mRNA and protein and Bcl-2 protein was down-regulated, while that of Bax and Bak proteins was up-regulated, and the activity of caspase-3 was elevated. Furthermore, the level of PGE2 was decreased transiently after 12 h of treatment with 30 μM troglitazone.
CONCLUSION: TGZ inhibits cell proliferation and induces apoptosis in HepG2 cells, which may be associated with the activation of caspase-3-like proteases, down-regulation of the expression of COX-2 mRNA and protein, Bcl-2 protein, the elevation of PGE2 levels, and up-regulation of the expressions of Bax and Bak proteins.
- Citation: Li MY, Deng H, Zhao JM, Dai D, Tan XY. PPARγ pathway activation results in apoptosis and COX-2 inhibition in HepG2 cells. World J Gastroenterol 2003; 9(6): 1220-1226
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1220.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1220
Peroxisome proliferator-activated receptors (PPARs) are transcription factors belonging to the nuclear receptor gene family. PPARs bind to specific response elements as heterodimers with the retinoid X receptor and activate transcription in response to a variety of endogenous and exogenous ligands, including some polyunsaturated fatty acids, arachidonic acid metabolites, and some anti-diabetic drugs and non-steroidal anti-inflammatory drugs[1-6]. Recently, PPARs subfamily has been defined as PPARα, PPARβ and PPARγ. Three PPAR isoforms differ in their tissue distribution and ligand specificity. PPARα is predominantly expressed in tissues exhibiting high catabolic rate of fatty acids, whereas PPARβ expression is ubiquitous, and its physiological role is not clear. PPARγ is expressed predominantly in adipose tissue, the adrenal gland, spleen, large colon and the immune system. Several lines of evidence indicate that PPARγ plays an important role in regulating adipocyte differentiation and glucose homeostasis. Both PPARα and PPARγ have been shown to be involved in anti-inflammatory reactions mediated by arachidonic acid metabolites. PPARα binds to, and is activated by leukotriene B4, and its level is regulated at the transcriptional level by anti-flammatory glucocorticoids[7-15]. PPARγ is activated by prostaglandin D2 metabolite 15-deoxy-Ä[12,14] prostaglandin J2(15 d-PGJ2) and synthetic anti-diabetic thiazolidinedione drugs, resulting in down-regulation of the expression of pro-inflammatory genes and inhibition of tumor cell growth[16,17].
Cyclooyxgenase (COX) is a rate-limiting enzyme, catalyzing the initial step in biosynthesis of prostaglandins (PGs) from arachidonic acid[18,19]. COX is encoded by two separate genes, COX-1 and COX-2, both of which participate in formation of a variety of eicosanoids including PGD2, PGE2, PGI2, PGF2α, and thromboxane A. COX-1 is expressed constitutionally in most tissues and has been proposed to be a house-keeping gene, which is involved in cytoprotection of gastric mucosa, vasodilation in kidney, and control of platelet aggregation. In contrast, COX-2 is an inducible immediate-early gene that is upregulated by various stimuli including mitogens, cytokines, growth factors, and tumor promoters. Previous studies have demonstrated that COX-2 expression is aberrantly increased in (various) human epithelial cancers in colorectum, esophagus, stomach, lung, and bladder[20-39]. These findings suggest that up-regulation of COX-2 may be a common mechanism in epithelial carcinogenesis. Recently, PPARγ ligands was found to suppress COX-2 expression in fetal hepatocytes[40] and in macrophage-like differentiated U937 cells[41]. However, other authors reported that 15d-PGJ2 induced the expression of COX-2 in immortalized epithelial[42] and colorectal cancer cells[43]. The mechanisms for the different regulation of COX-2 expression by PPARγ ligands remain to be elucidated. In the present study, we wanted to investigate the effect of PPARγ activation on cell growth and apoptosis, and to investigate underlying mechanism in regard to the expression of COX-2 and Bcl-2 members in HepG2 cells.
Human liver cancer cell line HepG2 was provided by the American Type Culture Collection. Cells were grown in RPMI-1640 medium supplemented with 15% new born bovine serum, penicillin G (100 kU•l-1) and kanamycin (0.1g/L) at 37 °C in the 5% CO2 incubator. Cells were grown on 96-well plates for MTT assay, [3H] thymidine incorporation and DNA fragmentation enzyme-linked immunosorbent assay (ELISA). For the experiment, cells were grown in fresh serum-free medium, incubated for 6 h, and treated with experimental reagents.
Cell growth was assessed by a modified MTT assay. About 2 × 105 cells/well were plated in 96-well microtiter plates and incubated overnight. Cells were then treated with troglitazone for 48 h in various concentrations. Then 10 mL stock MTT (0.5 g/L) was added to each well, and the cells were further incubated at 37 °C for 4 h. After supernatant was removed, 100 μL of 0.04 M HCl in isopropanol was added to each well to solubilize the formazan products. The absorbance at the wavelength of 570 nm was measured by a micro-ELISA reader. The negative control well contained medium only. The ratios of the absorbance of treated cells relative to those of the control wells were calculated and expressed as percentage of growth inhibition.
Cells were planted in 96-well plates and grown for 24 h after being starved by growing in the serum-free medium for 48 h. Then, they were treated with troglitazone for 48 h and labeled with 5 μCi of [3H] thymidine for 4 h. Radioactivity was detected using a Beckman L5 counter, after the reaction was washed and stopped with 5% trichloro acetic acid and the cells solubilized in 0.5% of 0.25 N sodium hydroxide. Each experiment was done in quadruplicates and repeated at least three times.
Cells were fixed with 4% formaldehyde in phosphate-buffered saline (PBS) for 10 min, stained by Hoechst 33258 (10 mg/L) for 1 hour, and subjected to fluorescence microscopy. After treatment with troglitazone, morphologic changes, including reduction in cell size and nuclear chromatin condensation, were observed.
After induction of apoptosis, cells (7 × 106/sample, both attached and detached cells) were lyzed with 150 μL hypotonic lysis buffer (edetic acid 10 mM, 0.5% Triton X-100, Tris-HCl, pH7.4) for 15 min on ice and were precipitated with 2.5% polyethylene glycol and 1 M NaCl for 15 min at 4 °C. After centrifugation at 16000 × g for 10 min at room temperature, the supernatant was treated with proteinase K (0.3 g/L) at 37 °C for 1 h and precipitated with isopropanol at -20 °C. After centrifugation, each pellet was dissolved in 10 μL of Tris-EDTA (pH7.6) and electrophoresed on a 1.5% agarose gel containing ethidium bromide. DNA ladder pattern was identified under ultraviolet light.
HepG2 cells were grown in 96-well plates. The cells were incubated with various dose of troglitazone for 48 h. DNA fragmentation was detected using an enzyme-linked immunosorbent assay (ELISA) kit (Roche). This assay was based on a quantitative sandwich enzyme-immunoassay directed against cytoplasmic histone-associated DNA fragments. Briefly, the cells were incubated in 200 μL of lysis buffer. After centrifugation, 20 mL of the supernatant was reacted overnight at 4 °C in streptavidin-coated wells with 80 μL of biotinylated anti-histone antibody and peroxidase-conjugated anti-DNA antibody. After washing, the immunocomplex-bound peroxidase was probed with 2, 2-azino-di[3-ethylbenzthiazoline sulfonate] for spectrophotometric detection at 405 nm.
TUNEL reaction was done using apoptosis detection system (Cayman). Cells were fixed overnight at 4 °C with 4% paraformaldehyde in PBS. The samples were washed three times with PBS and permeabilized by 0.2% Triton X-100 in PBS for 15 min on ice. After washed twice, cells were equilibrated at room temperature for 15 to 30 min in equilibration buffer (potassium cacodylate 200 mM, dithiothreitol 0.2 mM, bovine serum albumin 0.25 g/L, and cobalt chloride 2.5 mM in 25 mM Tris-HCl, pH6.6), and then incubated in a solution containing 5 μM pluorescein-12-dUTP, 10 μM dATP, 100 μM edetic acid, and terminal deoxynucleotidyl transferase at 37 °C for 1.5 h in a dark chamber. The tailing reaction was terminated by 2 × standard saline citrate (SSC). The samples were washed three times with PBS and analyzed by fluorescence microscopy. At least 1000 cells were counted, and the percentage of TUNEL-positive cells was determined.
After incubation with different doses of troglitazone for 6 h, cells were washed with RPMI. Total RNA was extrcted from adherent cells using Rneasy Mini kits (Sigma) as described previously[37]. 30 mg of total RNA from each sample was separated on agarose/formaldehyde gels and transferred to nylon membranes. The membrane was hybridized with probes for COX-2 and for GAPDH as a reference.
Total RNA was extracted from cells using TRIzolTM (Sigma). COX-2 and beta-actin mRNA were detected by polymerase-chain-reaction following reverse transcription- (RT-PCR) as described[37]. Primers for beta-actin were: sense 5'-ATCT-GGCACCACACCTTCTACAATGAGCTGCG-3', antisense 5'-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3'.
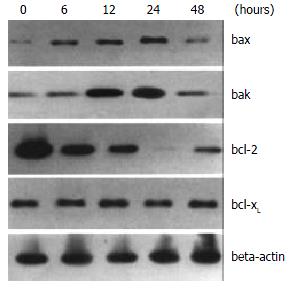
The cells were lysed in a lysis buffer (hepes 25 mM, Triton X-100 1.5%, sodium deoxycholate 1%, SDS 0.1%, NaCl 0.5 M, edetic acid 5 mM, NaF 50 mM, sodium vanadate 0.1 mM, phenylmethylsulfonyl fluoride (PMSF) 1 mM and leupeptin 0.1 g/L, pH7.8) at 4 °C with sonication. The lysates were centrifuged at 15000 g for 15 min and the concentration of the protein in each lysate was determined with Coomassie brilliant blue G-250. Loading buffer (42 mM Tris-HCl, containing 10% glycerol, 2.3% SDS, 5% 2-mercaptoethanol and 0.002% bromophenol blue) was then added to each lysate, which was subsequently boiled for 3 min and then electrophoresed on a SDS-polyacrylamide gel. Proteins were transferred onto a nitrocellulose filter and incubated separately with the antibodies against Bcl-2, Bax, Bak, Bcl-xL and COX-2, and then labeled with peroxidase-conjugated secondary antibodies. The reactions were visualized using the enhanced chemiluminescence reagent (Sigma). The results were approved by repeating the reactions 2 times.
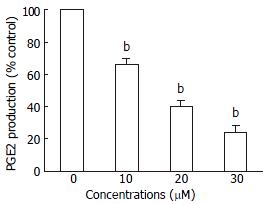
To determine the levels of PGE2, HepG2 cells were treated with different concentrations of troglitazone for 24 h. The quantity of PGE2 in supernatants was immediately determined with the PGE2 Enzyme Immunoassay kit (Caymen Chemical) according to the manufacturer's instructions. Data were recorded using a Dynatech MR50000 microplate reader and normalized to micrograms of protein.
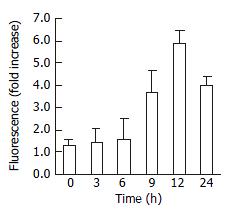
Caspase-3 activity was evaluated using a caspase assay kit following instructions of the manufacturer. In brief, caspase-3 fluorogenic substrate (Ac-DEVD-AMC or Ac-IETD-AMC) was incubated with JTE522-treated cell for 1 h at 37 °C, then AMC released from Ac-DEVD-AMC or Ac-IETD-AMC was detected using a fluorometric plate reader with an excitation wavelength of 380 nm and an emission wavelength of 420-460 nm.
Data were presented as the mean ± standard error, unless otherwise indicated. Multiple comparisons were examined for significant differences using analysis of variance, followed by individual comparisons with the Bonferroni post-test. Comparisons between two groups were made with the Student t test. P < 0.05 was considered significant.
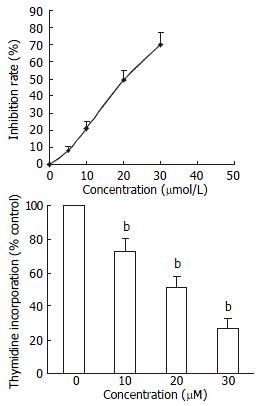
HepG2 cells were incubated with various does of trogliatzone for 48 h. MTT assay showed that trogliatzone significantly inhibited cell viability. The inhibition was dependent on dose of trogliatzone administered (Figure 1A). Application of trogliatzone also resulted in a reduction of [3H] thymidine uptake in a dose-dependent manner (Figure 1B).
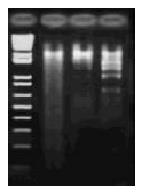
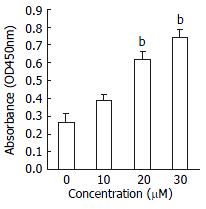
Effect of PPARγ activation on apoptosis was assessed by staining with Hoechst 33258, TUNEL reaction, DNAfragmentation demonstration on an agarose gel and by ELISA. The initiating effect of PPARγ activation on apoptosis was confirmed in HepG2 cell, the morphologic changes included reduction in cell size and nuclear chromation condensation visualized by Hoechst 33258 staining. The apoptotic index was also increased by treatment with different concentration of troglitazone from 3.2% ± 1.2% to 53% ± 2.6%. Agarose gel electrophoresis showed DNA ladder pattern in the exposed HepG2 cells (Figure 2). The PPARγ pathway-induced apoptosis was further demonstrated in quantitative measurement of cytoplasmic histone-associated DNA fragment by ELISA. As shown in Figure 3, troglitazone induced significant increase in DNA fragmentation in a dose-dependent manner.
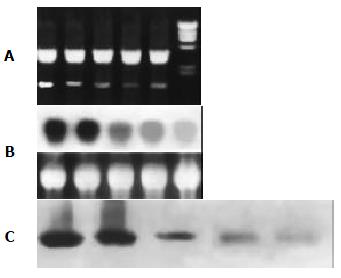
The fact that the COX-2 promoter contains a PPRE indicates that COX-2 might be one of the downstream targets of the PPARγ pathway. In the present study, COX-2 expression was observed in HepG2 cells treated with vehicle or 30 μM troglitazone. After 6, 12, 24 and 48 h of the treatment, cells were harvested. COX-2 mRNA was analysed by RT-PCR (4A) and Northern blotting (4B), and its translation product was demonstrated by Western blotting (4C). As shown in Figure 4, no significant change was detected during the first 6 h of treatment when compared with the control. After treatment with 30 μM troglitazone for 12 h, the expression of COX-2 was inhibited.
To further elucidate the mechanisms of troglitazone-induced apoptosis in HepG2 cells, we assessed the involvement of bcl-2 family proteins in the process by Western blotting. Expression of Bax protein was up-regulated 6 h after 20 mM troglitazone treatment and remained elevated to 24 h. Expression level of Bak protein was also elevated 12 h after the treatment with 30 μM troglitazone and declined at 48 h. On the controry, Bcl-2 protein expression was down-regulated at 6 h and undetectable at 24 h. No significant change was observed in the expression of bcl-xL protein (Figure 5).
The levels of PGE2 in vehicle controls were always high throughout the culture. When HepG2 cells were treated with 30 μM troglitazone, PGE2 concentration decreased transiently at 12 h (Figure 6).
In consideration of frequent involvement of caspaes activation in apoptosis, caspase-3 activity was assessed in HepG2 cell, treated with 20 μM troglitazone. As shown in Figure 7, the caspase-3 activity increased with the treatment, the reaction was time-dependent.
Potent effects of PPARγ on cell proliferation and cell cycling have been described. PPARγ ligands can trigger cell cycle arrest in NIH3T3 cells and HIB-1B cells[44]. PPARγ ligands can also induce terminal differentiation and withdrawal of human liposarcoma cells from the cell cycle[45]. Importantly, PPARγ ligands have been found to slow down the progression of advanced liposarcoma in humans[46]. Given the expression of PPARγ in nonadipose tissues, the effect of PPARγ on human breast cancer, gastric cancer, prostate cancer, colon cancer and transtitional cell, bladder cancer have been explored. Treatment of cultured breast cancer cells with troglitazone results in cell growth arrest and promotes differentiation[47]. Troglitazone has also been shown to inhibit tumor growth and induce apoptosis in human breast cancer cells in vitro and in BNX mice. Moreover, another PPARγ ligand, GW7845, has been shown to decrease tumor incidence, tumor growth and tumor burden I, the NMU induced mammary carcinoma[48]. These data suggest that PPARγ ligands may be used as novel, nontoxic and selective chemotherapeutic agents for human breast cancers. In the present study, our results have shown that activation of PPARγ by troglitazone inhibits cell growth and induces apoptosis in human liver cancer HepG2 cells. We confirmed that the induction of apoptosis was mediated through down-regulation of COX-2 and Bcl-2 expression, and up-regulation of Bax and Bak expression. The down-regulation of COX-2 was coincident with down-regulation of the production of PGE2. The activity of Caspase-3 was increased after treatment with 30 μM PPARγ ligand troglitazone in a time-dependent manner.
Meade et al[49] have demonstrated that COX-2 expression is enhanced by peroxisome proliferators, including some fatty acids, PGs and NSAIDs, as well as the prototypical peroxisome proliferator WY-14, 643, in mammary and colonic epithelial cells, presumably through PPARγ. Yang et al[50] showed that activation of PPAR pathway by ciglitazone induced apoptosis and inhibition of COX-2 expression in human colon cancer cells HT-29, but the result was not approved in an observation by Lefebvre et al[51]. Our data showed that PPARγ activation inhibited the expression of COX-2. The discrepancy may be caused by different cell types used in these groups.
Overexpression of COX-2 plays important roles in cell adhesion, apoptosis and angiogenesis. Numerous epidemiological studies suggest that use of nonsteroidal anti-inflammatory drugs (NSAIDs) decreases the incidence of gastrointestinal cancers and COX-2 is recognized as a major target of NSAIDs[52-64]. Inhibition of COX-2 by NSAIDs or COX-2 specific inhibitors causes cell death in cancer cells, indicating that COX-2 may be used as an important molecular target for prevention and therapy in gastrointestinal cancers[65-70]. The mechanism of COX-2 expression remains unclear. Subbaramaiah and colleagues have shown that PPARγ can inhibit COX-2 expression.
In the present study, the levels of PGE2 were decreased in a time-dependent manner after the treatment with 30 μM troglitazone, and were correlated with the change in COX-2 expression. This is in agreement with previous observations in other cell lines[71-73]. Thus, excessively synthesized PGE2 mediated by overexpression of COX-2 is believed to play an important role in neoplasma formation. Inhibition of COX-2 activity may at least partly explain the chemopreventative effect of activated PPAR pathway in human liver cancer.
Apoptosis is characterized by a series of distinct morphological and biochemical changes. Several apoptosis-related genes have been found. One group of apoptosis regulatory genes is the Bcl-2 family[74-79]. Of these genes, Bcl-2, Bcl-xL are antiapoptotic, whereas Bax, Bcl-xs, Bak, Bad and Bik are proapoptotic. In this study, overexpression of Bax and Bak, and suppression of the expression of Bcl-2 were found during the apoptosis induced by PPARγ activation. These data confirm the role of these proteins in troglitazone-induced apoptosis in HepG2 cells. In addition, the activity of caspase-3 was also found to be elevated during the apoptotic process induced by PPARγ activation.
In summary, we have shown that activation of PPARγ by troglitazone induces apoptosis in HepG2 cells through down-regulation of the expression of COX-2 and bcl-2, up-regulation of bax and bak, and activation of caspase-3. Consistent with other potential chemopreventive agents in human liver cancer model, we believe that COX-2, bak, bax, bcl-2 and caspase-3 play some roles in the process of PPARγ activation-induced apoptosis. These serve as potential targets for future drugs or therapies for prevention and treatment of liver cancer.
Edited by Su Q and Wang XL
| 1. | Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5354] [Cited by in RCA: 5298] [Article Influence: 143.2] [Reference Citation Analysis (0)] |
| 2. | Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1335] [Cited by in RCA: 1300] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 3. | Blumberg B, Evans RM. Orphan nuclear receptors--new ligands and new possibilities. Genes Dev. 1998;12:3149-3155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 235] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 700] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 5. | Murao K, Ohyama T, Imachi H, Ishida T, Cao WM, Namihira H, Sato M, Wong NC, Takahara J. TNF-alpha stimulation of MCP-1 expression is mediated by the Akt/PKB signal transduction pathway in vascular endothelial cells. Biochem Biophys Res Commun. 2000;276:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1402] [Cited by in RCA: 1411] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 7. | Wu GD. A nuclear receptor to prevent colon cancer. N Engl J Med. 2000;342:651-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci U S A. 1995;92:7921-7925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 508] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 9. | Fajas L, Fruchart JC, Auwerx J. PPARgamma3 mRNA: a distinct PPARgamma mRNA subtype transcribed from an independent promoter. FEBS Lett. 1998;438:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 232] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Fajas L, Auboeuf D, Raspé E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779-18789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 892] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 11. | Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000;448:121-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 357] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-gamma. Nature. 1998;396:377-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 273] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Moras D, Gronemeyer H. The nuclear receptor ligand-binding domain: structure and function. Curr Opin Cell Biol. 1998;10:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 589] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 14. | Leibowitz MD, Fiévet C, Hennuyer N, Peinado-Onsurbe J, Duez H, Bergera J, Cullinan CA, Sparrow CP, Baffic J, Berger GD. Activation of PPARdelta alters lipid metabolism in db/db mice. FEBS Lett. 2000;473:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Liang YC, Tsai SH, Tsai DC, Lin-Shiau SY, Lin JK. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001;496:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Kubota T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, Miyoshi I, Koeffler HP. Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 1998;58:3344-3352. [PubMed] |
| 17. | Takahashi N, Okumura T, Motomura W, Fujimoto Y, Kawabata I, Kohgo Y. Activation of PPARgamma inhibits cell growth and induces apoptosis in human gastric cancer cells. FEBS Lett. 1999;455:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 184] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063-1073. [PubMed] |
| 19. | Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 870] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 20. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276-1280. [PubMed] |
| 22. | Mohammed SI, Knapp DW, Bostwick DG, Foster RS, Khan KN, Masferrer JL, Woerner BM, Snyder PW, Koki AT. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. 1999;59:5647-5650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Murphy VJ, Yang Z, Rorison KA, Baldwin GS. Cyclooxygenase-2-selective antagonists do not inhibit growth of colorectal carcinoma cell lines. Cancer Lett. 1998;122:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Kusuhara H, Komatsu H, Sumichika H, Sugahara K. Reactive oxygen species are involved in the apoptosis induced by nonsteroidal anti-inflammatory drugs in cultured gastric cells. Eur J Pharmacol. 1999;383:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Tanaka K, Pracyk JB, Takeda K, Yu ZX, Ferrans VJ, Deshpande SS, Ozaki M, Hwang PM, Lowenstein CJ, Irani K. Expression of Id1 results in apoptosis of cardiac myocytes through a redox-dependent mechanism. J Biol Chem. 1998;273:25922-25928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Kim YB, Kim GE, Cho NH, Pyo HR, Shim SJ, Chang SK, Park HC, Suh CO, Park TK, Kim BS. Overexpression of cyclooxygenase-2 is associated with a poor prognosis in patients with squamous cell carcinoma of the uterine cervix treated with radiation and concurrent chemotherapy. Cancer. 2002;95:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Ferrandina G, Legge F, Ranelletti FO, Zannoni GF, Maggiano N, Evangelisti A, Mancuso S, Scambia G, Lauriola L. Cyclooxygenase-2 expression in endometrial carcinoma: correlation with clinicopathologic parameters and clinical outcome. Cancer. 2002;95:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Tang X, Sun YJ, Half E, Kuo MT, Sinicrope F. Cyclooxygenase-2 overexpression inhibits death receptor 5 expression and confers resistance to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human colon cancer cells. Cancer Res. 2002;62:4903-4908. [PubMed] |
| 29. | Staats PS. Pain management and beyond: evolving concepts and treatments involving cyclooxygenase inhibition. J Pain Symptom Manage. 2002;24:S4-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Lin DT, Subbaramaiah K, Shah JP, Dannenberg AJ, Boyle JO. Cyclooxygenase-2: a novel molecular target for the prevention and treatment of head and neck cancer. Head Neck. 2002;24:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Jiang XH, Lam SK, Lin MC, Jiang SH, Kung HF, Slosberg ED, Soh JW, Weinstein IB, Wong BC. Novel target for induction of apoptosis by cyclo-oxygenase-2 inhibitor SC-236 through a protein kinase C-beta(1)-dependent pathway. Oncogene. 2002;21:6113-6122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | He Q, Luo X, Huang Y, Sheikh MS. Apo2L/TRAIL differentially modulates the apoptotic effects of sulindac and a COX-2 selective non-steroidal anti-inflammatory agent in Bax-deficient cells. Oncogene. 2002;21:6032-6040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Kirkpatrick K, Ogunkolade W, Elkak A, Bustin S, Jenkins P, Ghilchik M, Mokbel K. The mRNA expression of cyclo-oxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) in human breast cancer. Curr Med Res Opin. 2002;18:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother. 2002;56:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 363] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 35. | Badawi AF, Habib SL, Mohammed MA, Abadi AA, Michael MS. Influence of cigarette smoking on prostaglandin synthesis and cyclooxygenase-2 gene expression in human urinary bladder cancer. Cancer Invest. 2002;20:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Gallo O, Masini E, Bianchi B, Bruschini L, Paglierani M, Franchi A. Prognostic significance of cyclooxygenase-2 pathway and angiogenesis in head and neck squamous cell carcinoma. Hum Pathol. 2002;33:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Li HL, Zhang HW, Chen DD, Zhong L, Ren XD, St-Tu R. JTE-522, a selective COX-2 inhibitor, inhibits cell proliferation and induces apoptosis in RL95-2 cells. Acta Pharmacol Sin. 2002;23:631-637. [PubMed] |
| 38. | Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NF-κB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J Gastroenterol. 2002;8:431-435. [PubMed] |
| 39. | Li HL, Chen DD, Li XH, Zhang HW, Lü JH, Ren XD, Wang CC. JTE-522-induced apoptosis in human gastric adenocarcinoma [correction of adenocarcinoma] cell line AGS cells by caspase activation accompanying cytochrome C release, membrane translocation of Bax and loss of mitochondrial membrane potential. World J Gastroenterol. 2002;8:217-223. [PubMed] |
| 40. | Callejas NA, Castrillo A, Boscá L, Martín-Sanz P. Inhibition of prostaglandin synthesis up-regulates cyclooxygenase-2 induced by lipopolysaccharide and peroxisomal proliferators. J Pharmacol Exp Ther. 1999;288:1235-1241. [PubMed] |
| 41. | Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPARgamma. J Biol Chem. 2000;275:28028-28032. [PubMed] |
| 42. | Meade EA, McIntyre TM, Zimmerman GA, Prescott SM. Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J Biol Chem. 1999;274:8328-8334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Chinery R, Coffey RJ, Graves-Deal R, Kirkland SC, Sanchez SC, Zackert WE, Oates JA, Morrow JD. Prostaglandin J2 and 15-deoxy-delta12, 14-prostaglandin J2 induce proliferation of cyclooxygenase-depleted colorectal cancer cells. Cancer Res. 1999;59:2739-2746. [PubMed] |
| 44. | Altiok S, Xu M, Spiegelman BM. PPARgamma induces cell cycle withdrawal: inhibition of E2F/DP DNA-binding activity via down-regulation of PP2A. Genes Dev. 1997;11:1987-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 276] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 45. | Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CD, Brun RP, Mueller E, Altiok S, Oppenheim H. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci U S A. 1997;94:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 509] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 46. | Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, Spiegelman BM, Singer S. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci U S A. 1999;96:3951-3956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 376] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, Fletcher C, Singer S, Spiegelman BM. Terminal differentiation of human breast cancer through PPAR gamma. Mol Cell. 1998;1:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 613] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 48. | Suh N, Wang Y, Williams CR, Risingsong R, Gilmer T, Willson TM, Sporn MB. A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999;59:5671-5673. [PubMed] |
| 49. | He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 808] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 50. | Yang WL, Frucht H. Activation of the PPAR pathway induces apoptosis and COX-2 inhibition in HT-29 human colon cancer cells. Carcinogenesis. 2001;22:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Lefebvre AM, Chen I, Desreumaux P, Najib J, Fruchart JC, Geboes K, Briggs M, Heyman R, Auwerx J. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med. 1998;4:1053-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 466] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 52. | Lednicer D. Tracing the origins of COX-2 inhibitors' structures. Curr Med Chem. 2002;9:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Kawabe A, Shimada Y, Uchida S, Maeda M, Yamasaki S, Kato M, Hashimoto Y, Ohshio G, Matsumoto M, Imamura M. Expression of cyclooxygenase-2 in primary and remnant gastric carcinoma: comparing it with p53 accumulation, Helicobacter pylori infection, and vascular endothelial growth factor expression. J Surg Oncol. 2002;80:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Kong G, Kim EK, Kim WS, Lee KT, Lee YW, Lee JK, Paik SW, Rhee JC. Role of cyclooxygenase-2 and inducible nitric oxide synthase in pancreatic cancer. J Gastroenterol Hepatol. 2002;17:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Liu CM, Hong CY, Shun CT, Hsiao TY, Wang CC, Wang JS, Hsiao M, Lin SK. Inducible cyclooxygenase and interleukin 6 gene expressions in nasal polyp fibroblasts: possible implication in the pathogenesis of nasal polyposis. Arch Otolaryngol Head Neck Surg. 2002;128:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Maitra A, Ashfaq R, Gunn CR, Rahman A, Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Wilentz RE. Cyclooxygenase 2 expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasia: an immunohistochemical analysis with automated cellular imaging. Am J Clin Pathol. 2002;118:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Carlton PS, Gopalakrishnan R, Gupta A, Liston BW, Habib S, Morse MA, Stoner GD. Piroxicam is an ineffective inhibitor of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus. Cancer Res. 2002;62:4376-4382. [PubMed] |
| 58. | Hoozemans JJ, Brückner MK, Rozemuller AJ, Veerhuis R, Eikelenboom P, Arendt T. Cyclin D1 and cyclin E are co-localized with cyclo-oxygenase 2 (COX-2) in pyramidal neurons in Alzheimer disease temporal cortex. J Neuropathol Exp Neurol. 2002;61:678-688. [PubMed] |
| 59. | Wei M, Wanibuchi H, Morimura K, Iwai S, Yoshida K, Endo G, Nakae D, Fukushima S. Carcinogenicity of dimethylarsinic acid in male F344 rats and genetic alterations in induced urinary bladder tumors. Carcinogenesis. 2002;23:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 60. | Sunayama K, Konno H, Nakamura T, Kashiwabara H, Shoji T, Tsuneyoshi T, Nakamura S. The role of cyclooxygenase-2 (COX-2) in two different morphological stages of intestinal polyps in APC(Delta474) knockout mice. Carcinogenesis. 2002;23:1351-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Wu T, Han C, Lunz JG, Michalopoulos G, Shelhamer JH, Demetris AJ. Involvement of 85-kd cytosolic phospholipase A(2) and cyclooxygenase-2 in the proliferation of human cholangiocarcinoma cells. Hepatology. 2002;36:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Wardlaw SA, Zhang N, Belinsky SA. Transcriptional regulation of basal cyclooxygenase-2 expression in murine lung tumor-derived cell lines by CCAAT/enhancer-binding protein and activating transcription factor/cAMP response element-binding protein. Mol Pharmacol. 2002;62:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Shah T, Ryu S, Lee HJ, Brown S, Kim JH. Pronounced radiosensitization of cultured human cancer cells by COX inhibitor under acidic microenvironment. Int J Radiat Oncol Biol Phys. 2002;53:1314-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Lew JI, Guo Y, Kim RK, Vargish L, Michelassi F, Arenas RB. Reduction of intestinal neoplasia with adenomatous polyposis coli gene replacement and COX-2 inhibition is additive. J Gastrointest. Surg. 2002;6:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Qiu DK, Ma X, Peng YS, Chen XY. Significance of cyclooxygenase-2 expression in human primary hepatocellular carcinoma. World J Gastroenterol. 2002;8:815-817. [PubMed] |
| 66. | Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483-487. [PubMed] |
| 67. | Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796-800. [PubMed] |
| 68. | Wang X, Lan M, Wu HP, Shi YQ, Lu J, Ding J, Wu KC, Jin JP, Fan DM. Direct effect of croton oil on intestinal epithelial cells and colonic smooth muscle cells. World J Gastroenterol. 2002;8:103-107. [PubMed] |
| 69. | Niu ZS, Li BK, Wang M. Expression of p53 and C-myc genes and its clinical relevance in the hepatocellular carcinomatous and pericarcinomatous tissues. World J Gastroenterol. 2002;8:822-826. [PubMed] |
| 70. | Chen Q, Yang GW, An LG. Apoptosis of hepatoma cells SMMC-7721 induced by Ginkgo biloba seed polysaccharide. World J Gastroenterol. 2002;8:832-836. [PubMed] |
| 71. | Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I). J Natl Cancer Inst. 1998;90:1529-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 381] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 72. | Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198-204. [PubMed] |
| 73. | Tjandrawinata RR, Dahiya R, Hughes-Fulford M. Induction of cyclo-oxygenase-2 mRNA by prostaglandin E2 in human prostatic carcinoma cells. Br J Cancer. 1997;75:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 175] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 74. | Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792-796. [PubMed] |
| 75. | Liu S, Wu Q, Ye XF, Cai JH, Huang ZW, Su WJ. Induction of apoptosis by TPA and VP-16 is through translocation of TR3. World J Gastroenterol. 2002;8:446-450. [PubMed] |
| 76. | Xu CT, Huang LT, Pan BR. Current gene therapy for stomach carcinoma. World J Gastroenterol. 2001;7:752-759. [PubMed] |
| 77. | Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796-800. [PubMed] |
| 78. | Hou L, Li Y, Jia YH, Wang B, Xin Y, Ling MY, Lü S. Molecular mechanism about lymphogenous metastasis of hepatocarcinoma cells in mice. World J Gastroenterol. 2001;7:532-536. [PubMed] |
| 79. | Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403-406. [PubMed] |