Published online Apr 15, 2003. doi: 10.3748/wjg.v9.i4.779
Revised: October 27, 2002
Accepted: November 4, 2002
Published online: April 15, 2003
AIM: To study the relative efficacy of cisapride, metoclopramide, domperidone, erythromycin and mosapride on gastric emptying (GE) and small intestinal transit (SIT) in morphine treated mice.
METHODS: Phenol red marker meal was employed to estimate GE and SIT in Swiss albino mice of either sex. The groups included were control, morphine 1 mg/kg (s.c. 15 min before test meal) alone or with (45 min before test meal p.o.) cisapride 10 mg/kg, metoclopramide 20 mg/kg, domperidone 20 mg/kg, erythromycin 6 mg/kg and mosapride 20 mg/kg.
RESULTS: Cisapride, metoclopramide and mosapride were effective in enhancing gastric emptying significantly (P < 0.001) whereas other prokinetic agents failed to do so in normal mice. Metoclopramide completely reversed morphine induced delay in gastric emptying followed by mosapride. Metoclopramide alone was effective when given to normal mice in increasing the SIT. Cisapride, though it did not show any significant effect on SIT in normal mice, was able to reverse morphine induced delay in SIT significantly (P < 0.001) followed by metoclopramide and mosapride.
CONCLUSION: Metoclopramide and cisapride are most effective in reversing morphine-induced delay in gastric emptying and small intestinal transit in mice respectively.
- Citation: Suchitra A, Dkhar S, Shewade D, Shashindran C. Relative efficacy of some prokinetic drugs in morphine-induced gastrointestinal transit delay in mice. World J Gastroenterol 2003; 9(4): 779-783
- URL: https://www.wjgnet.com/1007-9327/full/v9/i4/779.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i4.779
Opioids are effective analgesics for moderate to severe pain[1]. Morphine is the most commonly prescribed opioid agonist for the treatment of chronic pain[2,3]. However, when opioids are given to alleviate pain they cause undesirable gastrointestinal (GI) side effects namely nausea, vomiting and reduced gastrointestinal transit[4]. Reduced GI transit can cause gastroesophageal reflux disease, bloating and idiopathic constipation[5].
The pathophysiology of GI delay due to opioids has been well described. Possible etiologies include increased stationary segmentation, reduced peristalsis and depression of secretory activity[6]. Due to the paucity of the data to guide practitioners in the management of GI side effects of opioids much of the information is extrapolated from different patient populations experiencing the same symptoms due to different etiologies.
Use of opioid antagonists such as naloxone, nalmefene, for opioid-induced GI delay[7-10], is limited by their cost and tendency to cause withdrawal symptoms in the doses used[10,11]. Peripherally acting methyl naltrexone and ADL-2698 (Alvimopan) are in the stage of investigational[11].
Mosapride, cisapride, metoclopramide, erythromycin and domperidone are commonly used prokinetic agents. At present, the data supporting their relative efficacy in opioid induced GI delay are lacking. Further their distinct mechanism of action leaves a scope for variation in their efficacy. The present work attempts to study the relative efficacy of various prokinetic drugs such as cisapride, metoclopramide, domperidone, erythromycin and mosapride on small intestinal transit (SIT) and gastric emptying (GE) in morphine treated mice.
Randomly bred healthy adult Swiss albino mice of either sex weighing between 20-25 g were obtained from JIPMER animal house, Pondicherry. One week prior to the experimentation, six mice were housed in separate cages and had free access to food and water. The experiments were conducted between 09:00 AM and 1:00 PM. The animals were fed with the pellets obtained from Prestige Agro-industries, Aurangabad. The study was approved by the institutional animal ethics committee.
Morphine sulphate (Govt. opium and alkaloid works, Ghazipur), cisapride (Torrent Pharmaceuticals, Ahmedabad), metoclopramide hydrochloride (Ipca laboratories Ltd., Mumbai), domperidone (Torrent Pharmaceuticals, Ahmedabad ), erythromycin ethylsuccinate (Emil pharmaceutical industries Pvt Ltd, Thane), mosapride citrate (Alembic chemical works Co. Ltd. Vadodara) and Thiopentone sodium (Abbot laboratories, Mumbai) were used. Other chemicals used were of analytical grade. Carboxymethyl cellulose (1%, 0.2 ml) was used as vehicle to administer prokinetic drugs.
Phenol red indicator weighing 25 mg was dissolved in 50 ml of distilled water and filtered. The filtrate was heated to 70 °C and methylcellulose (0.75 g) was added to it with continuous stirring. The mixture was then cooled to 37 °C.
The standard method of phenol red marker meal as performed by earlier workers was employed[12,13]. Mice were deprived of food for 24 h prior to experimentation but had free access to water, and 0.5 ml of phenol red meal was administered with the aid of the oral feeding syringe. Animals were killed by cervical dislocation under i.v. thiopentone sodium anesthesia 15 min after the administration of the meal. Abdomen was opened and stomach was dissected out after careful ligation at the cardiac and pyloric ends, and was washed with normal saline. The stomach was cut into pieces and homogenized with 25 ml of 0.1 N NaOH. To this 5 ml homogenate 0.5 ml of trichloroacetic acid (20% w/v) was added and centrifuged at 3000 rpm for 20 minutes. To one ml of supernatant 4 ml of 0.5 N NaOH was added. The absorbance of this pink colored liquid was measured using spectrophotometer at 560 nm (Model: HITACHI 150-20). This correlates with the concentration of phenol red in the stomach, which in turn depends upon the gastric emptying. The percentage gastric emptying is derived as (1-X/Y) 100 where, X is absorbance of phenol red recovered from the stomach of animals sacrificed 15 minutes after test meal. Y is mean (n = 5) absorbance of phenol red recovered from the stomachs of control animals (killed at 0 min following test meal).
The small intestine was dissected out from the pylorus and ileocaecal junction and the point to which meal had traversed was secured with thread to avoid change in the length of the transit due to handling. The total length of the small intestine and distance traveled by phenol red meal was measured. The small intestinal transit (SIT) was calculated considering the distance traveled by phenol red meal divided by total length of the small intestine multiplied by 100. Small intestinal transit was expressed as mean ± sem. The observer was blinded for the drugs administered to the mice.
Control groups received the equal volumes of vehicle through corresponding routes. The groups included were control, morphine 1 mg/kg (s.c.), cisapride 10 mg/kg (p.o.), metoclopramide 20 mg/kg (p.o.), domperidone 20 mg/kg (p.o.), erythromycin 6 mg/kg (p.o.) and mosapride 20 mg/kg (p.o.). The doses were selected based on the earlier reports[14,17], recommended clinical doses[18,20] and prior pilot experiments. Cisapride, metoclopramide, domperidone, erythromycin and mosapride in the dose mentioned above were given alone 45 minutes before the administration of phenol red meal. Morphine (s.c.) was injected 15 minutes before the administration of the meal.
Statistical analysis was carried out using Graph pad (Prism) soft ware. One way analysis of variance (ANOVA) for small intestinal transit and gastric emptying was applied separately followed by bonferroni post-test for multiple comparisons. Values were expressed as mean ± sem. P < 0.05 was considered significant.
Since significant difference was not noticed among inter-day and animals receiving saline or 1% carboxymethyl cellulose, data from these groups were pooled to serve as control group. Similar results obtained from morphine (1 mg/kg s.c.) treated groups were pooled. Morphine in the dose of 1 mg/kg (s.c.) caused significant decrease (61%) in SIT (P < 0.001) and (43%) in gastric emptying (P < 0.05).
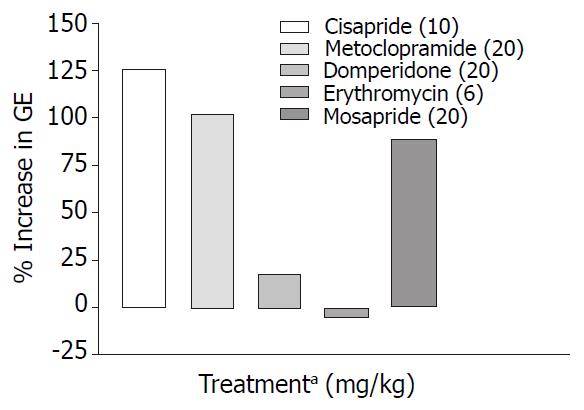
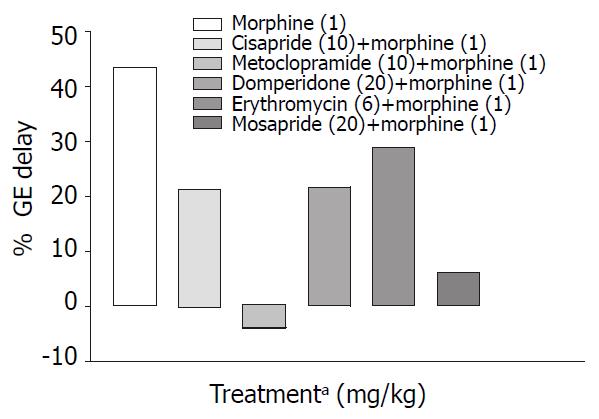
Cisapride, metoclopramide and mosapride in the doses employed increased gastric emptying significantly (P < 0.001) by 126%, 102%, 89% respectively compared with control animals, while domperidone and erythromycin failed to show any significant effect (Table 1, Figure 1). When combined with morphine (1 mg/kg s.c.) only metoclopramide and mosapride reversed morphine induced delay significantly (P < 0.05) (Table 2, Figure 2).
| Treatment1 (mg/kg) | GE (%) | SIT (%) |
| Control | 41.15 ± 3.76 | 51.11 ± 1.59 |
| Morphine(1) s.c. | 23.30 ± 2.83a | 19.73 ± 1.25b |
| Morphine (1) s.c. + | ||
| Cisapride (10) p.o. | 32.34 ± 2.52 | 46.28 ± 2.00d |
| Metoclopramide (20) p.o | 42.77 ± 2.04c | 34.01 ± 1.75d |
| Domperidone (20) p.o | 32.16 ± 3.48 | 28.63 ± 2.47 |
| Erythromycin (6) p.o | 29.39 ± 2.53 | 31.19 ± 1.08 |
| Mosapride (20) p.o | 38.59 ± 1.98c | 38.01 ± 1.61d |
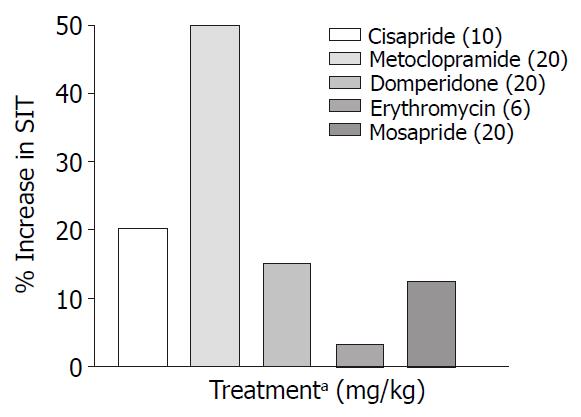
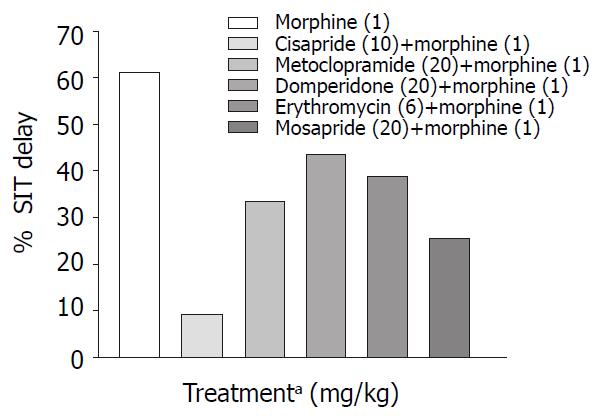
Only metoclopramide per se, significantly (P < 0.001) increased SIT by 50% (Figure 3). However, when combined with morphine (1 mg/kg, s.c.) cisapride showed maximum efficacy followed by mosapride and metoclopramide (Figure 4). Domperidone and erythromycin did not exhibit any significant effect (Table 2).
Opioids cause decrease in gastric emptying and intestinal motility, which leads to nausea, abdominal bloating and constipation[21]. Both exogenous and endogenous opioids inhibit propulsive movements of the intestine by interfering with the enteric regulation of propulsive motility[22]. They interact with µ and δ opioid receptors and cause decrease in cyclic AMP. Opioids increase the calcium dependent potassium conductance and thus, hyperpolarize myentric neurons, which reduce the entry of calcium during the action potential leading to decrease in intracellular calcium and thereby decreasing peristalsis[23].
In mice µ agonists administered subcutaneously produced dose-related inhibitions of gastrointestinal transit[24]. In our study there was significant decrease (61%) in SIT with morphine (1 mg/kg s.c.) compared with control animals (P < 0.001). Decrease in SIT seen with 1 mg/kg (s.c) of morphine is in agreement with earlier reports[14,16,17].
Prokinetic agents used to reverse morphine induced delay in GI transit showed variation in their efficacy. Cisapride (10 mg/kg, p.o.) though, did not show significant increase in SIT compared with control, it was most effective in reversing morphine induced delay in SIT (Figure 4). This finding is consistent with a clinical study where the effect of cisapride was significantly greater than that of metoclopramide[16]. Cisapride in the dose of 10 mg/kg (p.o.) showed significant increase in gastric emptying when compared with control animals but in morphine induced delay in gastric emptying it fails to show significant effect in overcoming the inhibition (Table 2).
Rectal administration of cisapride 30 mg 8th hourly had modest benefit in reversing small intestinal transit following intravenous meperidine in patients undergoing major abdominal surgery[25]. This may reflect an inability of cisapride to attain effective plasma concentrations when given rectally and this is supported by an another report where rectal cisapride was not able to overcome gastric stasis produced by morphine[26]. Above observations indicate that in morphine treated mice, cisapride is more effective in reversing morphine induced delay in SIT rather than delay in gastric emptying.
Similar differential effect of metoclopramide and cisapride on the abdominal surgery induced decrease in transit was seen in which metoclopramide further inhibited whereas cisapride ameliorated the inhibition of transit[27].
In our study metoclopramide (20 mg/kg p.o.) was found to be the most effective prokinetic agent when compared to control animals (Figure 3). Metoclopramide is a 5-HT4 agonist, 5-HT3 antagonist and antagonises the inhibitory effect of dopamine in the gastrointestinal tract. Despite above actions, it was only marginally effective in reversing morphine induced delay in SIT (Figure 2).
Metoclopramide in the dose of 20 mg/kg p.o. significantly increased gastric emptying when compared with controls (Table 1). In morphine induced gastric emptying delay, it reversed the morphine effect completely (Figure 2). In a clinical study conducted by McNeill et al[28], it was shown that i.v. metoclopramide antagonised the opioid premedication induced delay in gastric emptying but not i.m. metoclopramide. There is also evidence that the effectiveness of metoclopramide may depend on the route of administration[29,30].
Domperidone is a peripherally acting dopamine antagonist. Domperidone (20 mg/kg, p.o.) alone did not show any significant prokinetic effect as against control animals (Table 2). In our study, it was seen that in reversing morphine induced delay, though it could not significantly increase SIT, its efficacy was similar to that of metoclopramide (Figure 4). Since there is a risk of extrapyramidal effects with metoclopramide, domperidone may be useful in opioid induced delay in gastrointestinal transit where metoclopramide is contraindicated. Domperidone in the dose of 20 mg/kg p.o. did not show any significant increase in gastric emptying in normal and morphine treated animals (Table1 and Table 2).
Erythromycin, a motilinomimetic in the dose of 6 mg/kg (p.o.) did not show any significant prokinetic effect (Table 1) and had the least efficacy in reversing morphine induced delay in gastrointestinal transit (Table 2). Erythromycin in the dose of 1 mg/kg did not show any effect in postoperative ileus[27]. Erythromycin even at 40 mg/kg did not show any prokinetic effect in rats though motilin immunoreactivity has been demonstrated in the rat intestine[31]. The motilin receptor status of mouse upper GI tract is not documented. De Winter et al[27] suggested by their experiments that rat might not be the ideal species to test erythromycin. The fact that erythromycin was not effective in our study indicates that mice too may not be the ideal species for these type of studies.
Mosapride, a derivative of cisapride so far was not tested in morphine-induced delay in small intestinal transit. In our study though it did not show significant prokinetic effect in the dose (20 mg/kg p.o.) used, however it was less effective than 10 mg cisapride in reversing morphine induced delay in SIT. Efficacy of mosapride and metoclopramide in reversing morphine effect on GE cannot be differentiated from the available data.
A look at the SIT values (Table 1) indicates that metoclopramide has maximum efficacy in normal mice while cisapride is most efficacious in reversing morphine induced delay in SIT followed by metoclopramide and mosapride (Table 2). The above differences may be due to interactions taking place at various levels viz. the central and the peripheral nervous system. It is known that morphine suppresses the cholinergic outflow[32], while metoclopramide enhance cholinergic outflow[20] via 5-HT4 and D2 receptor interaction. It is also known that cisapride is non-selective 5-HT4 receptor agonist with affinity for D2, 5-HT2, α1 adrenergic and muscarinic receptors[33]. Further, mosapride a 5-HT4 agonist shows efficacy less than cisapride in reversing morphine effects on SIT and less than metoclopramide in GE. The intrinsic activity of mosapride was less than that observed for cisapride which indicates that it may act as a partial agonist[34]. Role of heterogenicity in 5-HT4 receptors in the differential effect of 5-HT4 agonists cannot be ruled out[35].
Mechanism underlying the stimulation of gastric and small intestinal motility by prokinetic agents is 5-HT4 receptor activation that involve cholinergic nerves. However the mechanism underlying the different profile of the effect of 5-HT4 agonists on gastric emptying and small intestinal transit remains unexplained.
Gastric emptying in normal mice was enhanced by cisapride, metoclopramide and mosapride (Figure 1) but in morphine treated mice metoclopramide was most effective followed by mosapride (Figure 2). This suggests that in morphine treated mice, 5-HT4 pathways may not play a major role. Effect of cisapride and metoclopramide on other receptors contributes predominantly in reversing the effect of morphine on SIT and gastric emptying respectively.
Results of this study and earlier reports indicate that efficacy of prokinetic agents is dependent on factors such as pathophysiology, species, site of gastrointestinal tract and pharmacological profile of the drug.
Morphine in the dose of 1 mg/kg (s.c.) inhibited the small intestinal transit by 61% and to a lesser extent, gastric emptying (43%) under the experimental conditions. Metoclopramide has significant prokinetic effect when compared with cisapride, domperidone, mosapride and erythromycin in normal mice. Metoclopramide, cisapride and mosapride enhance gastric emptying significantly compared to domperidone and erythromycin in normal mice. All the prokinetic agents used in our study differ in their prokinetic effects in counteracting the effect of morphine induced GI inertia. Cisapride is most effective in reversing morphine induced SIT delay followed by metoclopramide and mosapride. In morphine-induced GE delay metoclopramide is most effective followed by mosapride.
Edited by Ma JY and Xu XQ
| 1. | Barnett M. Alternative opioids to morphine in palliative care: a review of current practice and evidence. Postgrad Med J. 2001;77:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Moulin DE, Iezzi A, Amireh R, Sharpe WK, Boyd D, Merskey H. Randomised trial of oral morphine for chronic non-cancer pain. Lancet. 1996;347:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 269] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | McQuay H. Opioids in pain management. Lancet. 1999;353:2229-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 245] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Murphy DB, Sutton JA, Prescott LF, Murphy MB. Opioid-induced delay in gastric emptying: a peripheral mechanism in humans. Anesthesiology. 1997;87:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 171] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | McCallum RW. Review of the current status of prokinetic agents in gastroenterology. Am J Gastroenterol. 1985;80:1008-1016. [PubMed] |
| 6. | De Luca A, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther. 1996;69:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 208] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Meissner W, Schmidt U, Hartmann M, Kath R, Reinhart K. Oral naloxone reverses opioid-associated constipation. Pain. 2000;84:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Cheskin LJ, Chami TN, Johnson RE, Jaffe JH. Assessment of nalmefene glucuronide as a selective gut opioid antagonist. Drug Alcohol Depend. 1995;39:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Culpepper-Morgan JA, Inturrisi CE, Portenoy RK, Foley K, Houde RW, Marsh F, Kreek MJ. Treatment of opioid-induced constipation with oral naloxone: a pilot study. Clin Pharmacol Ther. 1992;52:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 126] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Yuan CS, Foss JF, O'Connor M, Toledano A, Roizen MF, Moss J. Methylnaltrexone prevents morphine-induced delay in oral-ce-cal transit time without affecting analgesia: a double-blind ran-domized placebo-controlled trial. Clin Pharmacol Ther. 1996;59:469-475. [RCA] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Schmidt WK. Alvimopan* (ADL 8-2698) is a novel peripheral opioid antagonist. Am J Surg. 2001;182:27S-38S. [PubMed] |
| 12. | Barquist E, Bonaz B, Martinez V, Rivier J, Zinner MJ, Taché Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am J Physiol. 1996;270:R888-R894. [PubMed] |
| 13. | Matsuda H, Li Y, Yamahara J, Yoshikawa M. Inhibition of gastric emptying by triterpene saponin, momordin Ic, in mice: roles of blood glucose, capsaicin-sensitive sensory nerves, and central nervous system. J Pharmacol Exp Ther. 1999;289:729-734. [PubMed] |
| 14. | Fickel J, Bagnol D, Watson SJ, Akil H. Opioid receptor expression in the rat gastrointestinal tract: a quantitative study with comparison to the brain. Brain Res Mol Brain Res. 1997;46:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Rowbotham DJ, Nimmo WS. Effect of cisapride on morphine-induced delay in gastric emptying. Br J Anaesth. 1987;59:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Bianchi G, Ferretti P, Recchia M, Rocchetti M, Tavani A, Manara L. Morphine tissue levels and reduction of gastrointestinal transit in rats. Correlation supports primary action site in the gut. Gastroenterology. 1983;85:852-858. [PubMed] |
| 17. | Patil CK, Kulkarni SK. Effect of physostigmine and cisapride on morphine-induced delayed gastric transit in mice. Ind J Pharmacol. 2000;32:321-323. |
| 18. | Brogden RN, Carmine AA, Heel RC, Speight TM, Avery GS. Domperidone. A review of its pharmacological activity, pharmacokinetics and therapeutic efficacy in the symptomatic treatment of chronic dyspepsia and as an antiemetic. Drugs. 1982;24:360-400. [PubMed] |
| 19. | Pandolfino JE, Howden CW, Kahrilas PJ. Motility-modifying agents and management of disorders of gastrointestinal motility. Gastroenterology. 2000;118:S32-S47. [PubMed] |
| 20. | Murphy DB, Sutton JA, Prescott LF, Murphy MB. Opioid-induced delay in gastric emptying: a peripheral mechanism in humans. Anesthesiology. 1997;87:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 171] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Harrington RA, Hamilton CW, Brogden RN, Linkewich JA, Romankiewicz JA, Heel RC. Metoclopramide. An updated review of its pharmacological properties and clinical use. Drugs. 1983;25:451-494. [PubMed] |
| 22. | Wong CL. Central and peripheral inhibitory effects of morphine on intestinal transit in mice. Methods Find Exp Clin Pharmacol. 1986;8:479-483. [PubMed] |
| 23. | Pasricha PJ. Prokinetic agents, antiemetics and agents used in irritable bowel syndrome. In Hardman JG, Limbard LE, eds The pharmacological basis of therapeutics. McGraw-Hill, New York, 2001:1021-1036. . |
| 24. | Pol O, Ferrer I, Puig MM. Diarrhea associated with intestinal inflammation increases the potency of mu and delta opioids on the inhibition of gastrointestinal transit in mice. J Pharmacol Exp Ther. 1994;270:386-391. [PubMed] |
| 25. | Benson MJ, Roberts JP, Wingate DL, Rogers J, Deeks JJ, Castillo FD, Williams NS. Small bowel motility following major intra-abdominal surgery: the effects of opiates and rectal cisapride. Gastroenterology. 1994;106:924-936. [PubMed] |
| 26. | Kluger MT, Plummer JL, Owen H. The influence of rectal cisapride on morphine-induced gastric stasis. Anaesth Intensive Care. 1991;19:346-350. [PubMed] |
| 27. | De Winter BY, Boeckxstaens GE, De Man JG, Moreels TG, Schuurkes JA, Peeters TL, Herman AG, Pelckmans PA. Effect of different prokinetic agents and a novel enterokinetic agent on postoperative ileus in rats. Gut. 1999;45:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | McNeill MJ, Ho ET, Kenny GN. Effect of i.v. metoclopramide on gastric emptying after opioid premedication. Br J Anaesth. 1990;64:450-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Bateman DN, Kahn C, Davies DS. Concentration effect studies with oral metoclopramide. Br J Clin Pharmacol. 1979;8:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Bateman DN, Kahn C, Mashiter K, Davies DS. Pharmacokinetic and concentration-effect studies with intravenous metoclopramide. Br J Clin Pharmacol. 1978;6:401-407. |
| 31. | Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, Poitras P. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G948-G952. |
| 32. | Cherubini E, Morita K, North RA. Opioid inhibition of synaptic transmission in the guinea-pig myenteric plexus. Br J Pharmacol. 1985;85:805-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Briejer MR, Akkermans LM, Schuurkes JA. Gastrointestinal prokinetic benzamides: the pharmacology underlying stimulation of motility. Pharmacol Rev. 1995;47:631-651. [PubMed] |
| 34. | Yoshida N. [Pharmacological effects of the gastroprokinetic agent mosapride citrate]. Nihon Yakurigaku Zasshi. 1999;113:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Mine Y, Yoshikawa T, Oku S, Nagai R, Yoshida N, Hosoki K. Comparison of effect of mosapride citrate and existing 5-HT4 receptor agonists on gastrointestinal motility in vivo and in vitro. J Pharmacol Exp Ther. 1997;283:1000-1008. [PubMed] |