Published online Apr 15, 2003. doi: 10.3748/wjg.v9.i4.701
Revised: August 27, 2002
Accepted: September 4, 2002
Published online: April 15, 2003
AIM: To investigate the anti-cancer effect and the immunological mechanism of ultrasound-guided intratumoral injection of Chinese medicine “Star-99” in hepatocellular carcinoma (HCC) of nude mice.
METHODS: Twenty-eight human hepatocellular carcinoma SMMC-7721 transplanted nude mice, 14 of hypodermically implanted and 14 of orthotopic liver transplanted, were randomly divided into three groups of which 14 mice with Star-99, and 7 with ethanol and saline respectively. Ten days after the transplantation the medicines were injected into the tumors of all the nude mice once every 5 d. After 4 injections the nude mice were killed. The diameters of three dimension of the tumors were measured by high frequency ultrasound before and after the treatment and the tumor growth indexes* (TGI) were calculated. Radioimmunoassay was used to detect the serum levels of interleukin-2 (IL-2) and tumor necrosis factor (TNF)-alpha. The tumor tissues were sent for flow cytometry (FCM) DNA analysis. Apoptotic cells were visualized by TUNEL assay. All the experiments were carried out by double blind method.
RESULTS: The TGI of Star-99 group (0.076 ± 0.024) was markedly lower than that of the saline group (4.654 ± 1.283) (P < 0.01). It also seemed to be lower than that of the ethanol group (0.082 ± 0.028), but not significantly different (P > 0.05). Serum levels of IL-2 and TNF-α were markedly higher than those of ethanol group and saline groups (P < 0.05). The mean apoptotic index (AI: percentage of TUNEL signal positive cells) in Star-99 group (48.98% ± 5.09%) was significantly higher than that of the ethanol group (11.95% ± 2.24%) and the saline group (10.48% ± 3.85%) (P < 0.01). FCM DNA analysis showed that the appearance rate of the apoptosis peak in Srar-99 group was 92.9%, markedly higher than that of the ethanol group (14.3%) and the saline group (0.0%) (P < 0.01). Correlation (r = 0.499, P < 0.05) was found between AI and serum level of TNF-α.
CONCLUSION: Star-99 has an effect on the elevation of the serum levels of IL-2 and TNF-α. It indicates that Star-99 has the function of enhancing the cellular immunity and inducing cancer cell apoptosis. The correlation between AI and serum level of TNF-α indicates that the elevation of the serum of TNF-α induced by Star-99 may be an important factor in the promotion of the hepatic cancer cell apoptosis. Star-99 has strong effects on the inhibition and destruction of cancer cells. Its curative effect is as good as ethanol. Its major mechanisms can be as follows: (1) it increases the serum levels of IL-2 and TNF-α and triggers cellular immunity. (2) It can induce cancer cells apoptosis, the effective mechanism of the Star-99 is different from that of the ethanol. The mechanisms of triggering the immunologic function of the organism and inducing cell apoptosis are, of particular significance. This study will provide a new pathway of drug administration and an experimental basis for the treatment of HCC with Chinese herbal, and the study of Star-99 in the treatment of tumor is of profound significance with good prospects.
- Citation: Lin LW, Lin XD, He YM, Gao SD, Xue ES. Experimental study on ultrasound-guided intratumoral injection of “Star-99” in treatment of hepatocellular carcinoma of nude mice. World J Gastroenterol 2003; 9(4): 701-705
- URL: https://www.wjgnet.com/1007-9327/full/v9/i4/701.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i4.701
The ultrasound-guided percutaneous intratumoral injection with ethanol, in treatment of hepatocellular carcinoma (HCC), has been widely used in the clinic in recent years since the report by the Japanese scholars (1983)[1-3]. Yet there are certain limitations in this treatment that lead us to search for newer drug that we have been studied for many years. In 1999, we discovered that the compound Chinese medicine “Star-99” had anti-cancer effect. In order to probe the effective mechanism of the ultrasound-guided intratumoral injection of HCC with Star-99, we detected the serum levels of interleukin-2 (IL-2) and tumor necrosis factor (TNF)-alpha, and conducted FCM DNA analysis and TUNEL assay to observe the phenomenon of apoptosis and to appraise the biological effect of Star-99.
For the experiment, we used 28 BALB/CA nude mice, which were 5-8 weeks old, provided by the Medical Experimental Animal Unit of Anti-cancer Center of Xia Men University, China. The average weight of the nude mice was 18 ± 2.1 grams. The mice were raised in the layer drift shelves under aseptic condition. The cages, cushion, drinking water and standard forage were provided by Shanghai Bikai Company periodically.
Human HCC SMMC-7721 cellular cultural suspension was centrifuged and the supernatant was removed. Then the cellular cultural liquid was added to make up 5 × 107 cells per milliliter. Subcutaneous injection was taken in the back of a nude mouse with 0.2 ml liquid. When the tumor grew to 1 cm in diameter, part of it was taken out under the aseptic condition and cut into small pieces in the size of 0.2 × 0.2 × 0.2 cm, which were transplanted subcutaneously at the back of other nude mice with a canula needle and underwent passages from generation to generation. The nude mice, under general anesthesia with sodium pentothal injected intraperitoneally. The process was as follows: The abdomen of the mouse was incised 1 cm in length deep to peritoneum on the linea alba below the xiphoid process. The liver was squeezed out, a piece of tumor was embedded into the liver with canula needle, the needle aperture was blocked with gelatin sponge to stop bleeding, then the liver was sent back into the abdomen, the peritoneum and skin were sutured and the process of orthotopic liver transplantation was completed.
Twenty-eight human HCC transplanted nude mice, including 14 implanted hypodermically and the other 14 with liver transplanted orthotopically, were randomly divided into three groups: the Star-99 group containing 14 nude mice, the ethanol group and the saline group containing 7 nude mice each. The three dimensional diameters of the tumor were measured by high frequency ultrasound (Aloka 5500 with 10 MHz probe) after 10 d transplantation of HCC. Then 0.1 ml Star-99, ethanol and saline were respectively injected into the center of the tumor with No.5 needle. The nude mice were killed after being injected every 5 d for a total of 4 injections in each mouse. The three dimensional diameters of the tumor were measured again before the mice were killed. Mice blood was obtained from the eye socket, and the sera were separated by low velocity centrifugation. The Radioimmunoassay (RIA) kit was purchased from Beijing East Asian Institute of Immunology. Sn-682 Radioimmunoassay γ counting apparatus was used to detect the serum levels of IL-2 and TNF-α. The apoptotic cells were visualized by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labelling (TUNEL). The TUNEL kit (POD) was purchased from Roche Molecular Biochemicals. The assay performance followed the technical manual and the references[4-7]. It was TUNEL positive when the nucleolus appeared evidently brown granule. Ten high power fields were observed in each slide and at least 1000 positive cells were calculated at random. The cellular suspension made by fresh tumor tissues was analyzed by FCM DNA. The appearance rates of the heteroploidy peak and apoptosis peak were analyzed. Double blind method was applied in this experiment.
The tumor growth index (TGI) was calculated by the formula: volume of tumor (after treatment-before treatment)/volume of tumor (before treatment). The serum levels of IL-2 and TNF-α were determined. The apoptotic index (AI: percentage of TUNEL signal positive cells) were calculated as the TUNEL-positive cells/the total number of cancer cells × 100%. The appearance rates of the heteroploidy peak and apoptosis peak of the tumor tissue were analyzed by FCM DNA. The correlation coefficient between AI and serum levels of TNF-α was calculated.
The results were expressed as the mean ± SD. The data were analyzed by one-way ANOVA with Student’s t test, chi-square test and liner regression analysis. Value of P < 0.05 was considered significant.
Table 1 showed that the growth index of Star-99 group was markedly lower than that of the saline group (P < 0.01). It was also lower than that of the ethanol group, but there was no significant difference between them (P > 0.05).
| Groups | n | x- | SD | P |
| Star-99 (A) | 14 | 0.076 | ± 0.024 | A, B > 0.05 |
| Ethanol (B) | 7 | 0.082 | ± 0.028 | B, C < 0.01 |
| Saline (C) | 7 | 4.654 | ± 1.283 | A, C < 0.01 |
Table 2 showed that the serum levels of IL-2 and TNF-α in the Star-99 group were 6.63 ± 1.39 ng/mL and 3.98 ± 1.05 ng/mL, markedly higher than those of the ethanol and the saline groups (P < 0.05).
| Groups | n | IL-2 (ng/mL) | TNF-α (ng/mL) | P |
| Star-99 (A) | 14 | 6.63 ± 1.39 | 3.98 ± 1.05 | A:B < 0.05 |
| Ethanol (B) | 7 | 4.22 ± 1.23 | 2.95 ± 1.01 | A:C < 0.05 |
| Saline (C) | 7 | 4.51 ± 0.84 | 2.84 ± 1.05 | B:C > 0.05 |
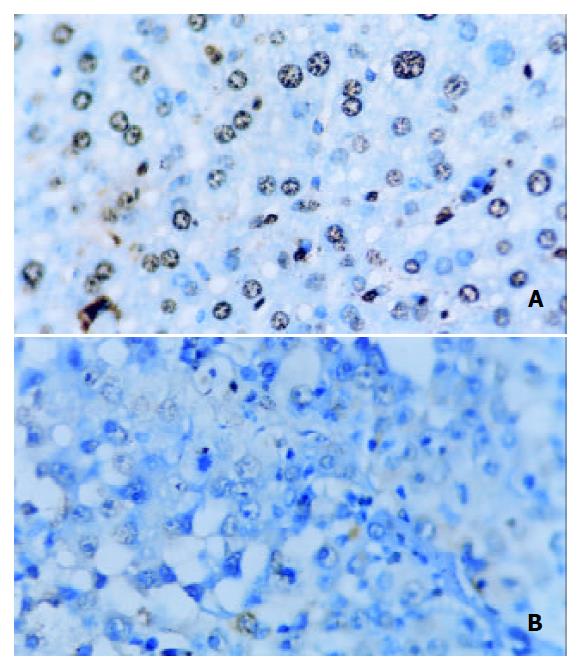
Table 3 showed that the AI in Star-99 group (48.98% ± 5.09%) was significantly higher than that of the ethanol group (11.95% ± 2.24%) and the saline group (10.48% ± 3.85%) (P < 0.01), (Figure 1).
| Groups | n | AI (%) | P |
| Star-99 (A) | 14 | 48.98 ± 5.09 | A:B < 0.01 |
| Ethanol (B) | 7 | 11.95 ± 2.24 | B:C > 0.05 |
| Saline (C) | 7 | 10.48 ± 3.85 | A:C < 0.01 |
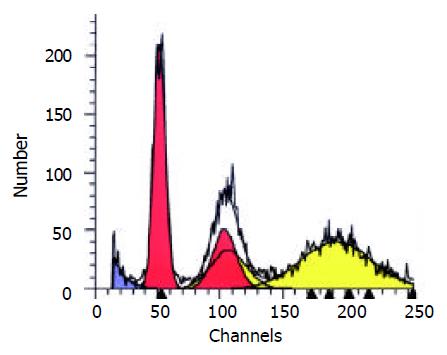
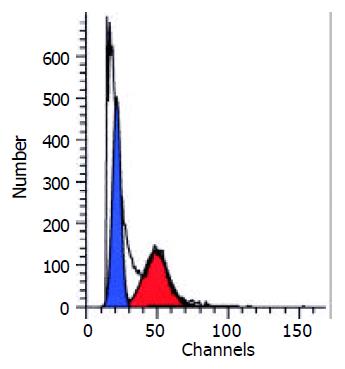
Table 4 showed the results of FCM DNA analysis of the tumor tissues of the three groups. The appearance rate of the heteroploidy peak after the treatment was 57.1% in the saline group (Figure 2), markedly higher than that of the ethanol group (0.0%) and the Star-99 group (7.1%) (P < 0.01). Another remarkable characteristic was that 92.9% of the Star-99 group appeared apoptosis peaks formed by the cells in the sub G1 period (Figure 3), markedly higher than that of the ethanol group and the saline group, which were 14.3% (1/7) and 0.0 (0/7), respectively (P < 0.01).
| Groups | n | Hereroploidy peak (%) | P | Apoptosis peak (%) | P |
| Star-99 (A) | 14 | 7.1 (1/14) | A:B > 0.05 | 92.9 (13/14) | A:B < 0.01 |
| Ethanol (B) | 7 | 0.0 (0/7) | B:C < 0.05 | 14.3 (1/7) | B:C > 0.05 |
| Saline (C) | 7 | 57.1 (4/7) | A:C < 0.05 | 0.0 (0/7) | A:C < 0.01 |
There was close relationship between HCC apoptosis and serum level of TNF-α. Significant correlation (r = 0.499, P < 0.05) was found between AI and serum levels of TNF-α.
At present, many kinds of ultrasound-guided interventional therapy have been practiced by clinicians such as laser[8,9], microwave[10-13], radio frequency[14-19] and high energy focus ultrasound[20,21] since 1990s. The efficacy are as good as that of surgical resection[2,22-24]. Early In 1985, the American National Cancer Institute considered biotherapy as the fourth modality in cancer treatment as it attacked the tumor cells directly; furthermore, it stimulates the immune system of the host. The present study provided a new avenue to combat the tumor from immunologic point of view. As shown in this experiment, the Star-99 has marked effect on the growth restraint of the tumor, the effectiveness is more or less equivalent to that of the ethanol.
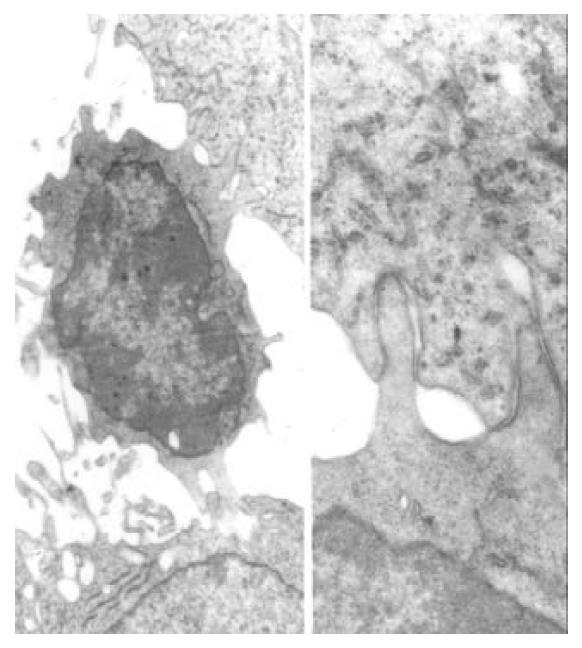
The two important cytokines IL-2 and TNF-α can be used as biological response modifiers (BRM). IL-2 can enhance the activity of lymphocyte and the killing effect of CTL, NK and LAK cells, induce secretion of cytokine. The serum level of IL-2 reflects the host immunologic function to a certain degree[25]. In this experiment, the serum level of IL-2 in the Star-99 group was 6.63 ± 1.39 ng/mL, markedly higher than those of ethanol and saline groups (P < 0.05). In another experiment of this study, the electron microscopy showed many lymphocytes in the tumor tissues of the Star-99 group[26]. Figure 4 showed the microvilli on the surface of the lymphocytes attacked the cancer cells. The membrane of tumor cell in contact with the sensitized T lymphocyte became broken with the organelle and the nucleus dissolved and the vacuolation of the cytoplasm as well. This phenomenon further illustrate that the Star-99 could stimulate or induce the cellular immunity function of the organism. TNF-α is mainly produced by macrophage and activated T lymphocyte which can inhibit or destroy the cancer cells. It can also induce apoptosis by combining with TNF receptor and resulting in chain reactions[27,28]. In the Star-99 group, the serum level of TNF-α was 3.98 ± 1.05 ng/mL, markedly higher than those of ethanol and saline groups. Significant correlation (r = 0.499, P < 0.05) was found between AI and serum levels of TNF-α. It indicated that the elevation of the serum TNF-α induced by Star-99 might be an important factor in the promotion of the HCC cells apoptosis.
Cancer growth not only depends on proliferation[29-35] but also on the reduced apoptosis[36-39]. At present time, cancer therapy is now focusing on promoting apoptosis[40,41]. The apoptotic cells could be identified by TUNEL assay[5,42]. In this experiment, the apoptotic index in Star-99 group (48.98% ± 5.09%) was significantly higher than that in the ethanol group (11.95% ± 2.24%) and the saline group (10.48% ± 3.85%) (P < 0.01), which was consistent with the result of FCM DNA analysis, the appearance rate of the apoptosis peak in Srar-99 group was 92.9%, markedly higher than that of the ethanol group (14.3%) and the saline group (0.0%) (P < 0.01). These results showed that although the cancer cells in ethanol group were destroyed which was different from that by means of apoptosis. In the Star-99 group it was mainly by induction of apoptosis.
The results of this study illustrated that the compound Chinese medicine Star-99 has the strong effect on the growth restraint of the tumor. Apart from its direct destruction of cancer cells, the mechanism of the efficacy may be due to: (1) It can increase the serum levels of IL-2 and TNF-α and trigger the cellular immunity. (2) It can induce apoptosis of the cancer cells. The anti-cancer effect of Star-99 is more or less equivalent to the ethanol, but by different mechanisms. Based on the above findings, Star-99 may have great significance and good prospects in the future treatment of HCC.
Edited by Wu XN
| 1. | Lin LW, Lin XY, He YM, Gao SD, Xue ES, Lin XD, Yu LY. Experimental and clinical assessment of percutaneous hepatic quantified ethanol injection in treatment of hepatic carcinoma. World J Gastroenterol. 2004;10:3112-3117. [PubMed] |
| 2. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 540] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 3. | Livraghi T, Benedini V, Lazzaroni S, Meloni F, Torzilli G, Vettori C. Long term results of single session percutaneous ethanol injection in patients with large hepatocellular carcinoma. Cancer. 1998;83:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483-487. [PubMed] |
| 5. | Li J, Wang WL, Wang WY, Liu B, Wang BY. Apoptosis in human hepatocellular carcinoma by terminal deoxynucleotidyl trans-ferase-mediate. Huaren Xiaohua Zazhi. 1998;6:491-494. |
| 6. | Wang LS, Pan LJ, Li MS, Sun Y, Shi L, Zhang YL, Zhou DY. Apoptosis of large bowel carcinoma induced by complete pep-tide polyose. Shijie Huaren Xiaohua Zazhi. 1999;7:710. |
| 7. | Zhang Z, Yuan Y, Gao H, Dong M, Wu HQ, Wang L, Wang MX. In situ observation of apoptosis and proliferation in gas-tric cancer and precanceration. Shijie Huaren Xiaohua Zazhi. 1999;7:802-803. |
| 8. | Pacella CM, Bizzarri G, Magnolfi F, Cecconi P, Caspani B, Anelli V, Bianchini A, Valle D, Pacella S, Manenti G. Laser thermal ablation in the treatment of small hepatocellular carcinoma: results in 74 patients. Radiology. 2001;221:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Pacella CM, Bizzarri G, Cecconi P, Caspani B, Magnolfi F, Bianchini A, Anelli V, Pacella S, Rossi Z. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Lu MD, Chen JW, Xie XY, Liu L, Huang XQ, Liang LJ, Huang JF. Hepatocellular carcinoma: US-guided percutaneous microwave coagulation therapy. Radiology. 2001;221:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Ishida T, Murakami T, Shibata T, Inoue Y, Takamura M, Niinobu T, Sato T, Nakamura H. Percutaneous microwave tumor coagulation for hepatocellular carcinomas with interruption of segmental hepatic blood flow. J Vasc Interv Radiol. 2002;13:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Dick EA, Taylor-Robinson SD, Thomas HC, Gedroyc WM. Ablative therapy for liver tumours. Gut. 2002;50:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Matsuda M, Fujii H, Kono H, Matsumoto Y. Surgical treatment of recurrent hepatocellular carcinoma based on the mode of recurrence: repeat hepatic resection or ablation are good choices for patients with recurrent multicentric cancer. J Hepatobiliary Pancreat Surg. 2001;8:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 501] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Ahmed M, Lobo SM, Weinstein J, Kruskal JB, Gazelle GS, Halpern EF, Afzal SK, Lenkinski RE, Goldberg SN. Improved coagula-tion with saline solution pretreatment during radiofrequency tumor ablation in a canine model. J Vasc Interv Radiol. 2002;13:717-724. [RCA] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Choi D, Lim HK, Kim SH, Lee WJ, Jang HJ, Kim H, Lee SJ, Lim JH. Assessment of therapeutic response in hepatocellular carcinoma treated with percutaneous radio frequency ablation: comparison of multiphase helical computed tomography and power Doppler ultrasonography with a microbubble contrast agent. J Ultrasound Med. 2002;21:391-401. [PubMed] |
| 17. | Giorgio A, Francica G, Tarantino L, de Stefano G. Radio-frequency ablation of hepatocellular carcinoma lesions. Radiology. 2001;218:918-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol. 2001;12:1135-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 305] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 741] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 20. | McDannold NJ, Jolesz FA, Hynynen KH. Determination of the optimal delay between sonications during focused ultrasound surgery in rabbits by using MR imaging to monitor thermal buildup in vivo. Radiology. 1999;211:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Wu F, Chen WZ, Bai J. Effect of high-intensity focused ultrasound on the patients with hepatocellular carcinoma: preliminary report. Zhonghua Chaosheng Yingxiangxue Zazhi. 1999;8:213-216. |
| 22. | Yamamoto J, Okada S, Shimada K, Okusaka T, Yamasaki S, Ueno H, Kosuge T. Treatment strategy for small hepatocellular carcinoma: comparison of long-term results after percutaneous ethanol injection therapy and surgical resection. Hepatology. 2001;34:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 851] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 24. | Morimoto M, Sugimori K, Shirato K, Kokawa A, Tomita N, Saito T, Tanaka N, Nozawa A, Hara M, Sekihara H. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology. 2002;35:1467-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Parslow TG, Stites DP, Terr AI, Imboden JB. Medical Immunology. Beijing science Press. 2002;154-156. |
| 26. | Lin LW, He YM, Gao SD, Yan FD. Percutaneous intratumoral injection of chinese traditional medicine "Star-99" in treatment of hepatic carcinoma: an experimental study. Zhonghua Chaosheng Yingxiangxue Zazhi. 2002;11:45-48. |
| 27. | Kimura K, Bowen C, Spiegel S, Gelmann EP. Tumor necrosis factor-alpha sensitizes prostate cancer cells to gamma-irradiation-induced apoptosis. Cancer Res. 1999;59:1606-1614. [PubMed] |
| 28. | Liang WJ, Huang ZY, Ding YQ, Zhang WD. Lovo cell line apoptosis induced by cycloheximide combined with TNFα. Shijie Huaren Xiaohua Zazhi. 1999;7:326-328. |
| 29. | Shen YF, Zhuang H, Shen JW, Chen SB. Cell apoptosis and neoplasms. Shijie Huaren Xiaohua Zazhi. 1999;7:267-268. |
| 30. | He SW, Shen KQ, He YJ, Xie B, Zhao YM. Regulatory effect and mechanism of gastrin and its antagonists on colorectal carcinoma. World J Gastroenterol. 1999;5:408-416. [PubMed] |
| 31. | Huang PL, Zhu SN, Lu SL, Dai ZS, Jin YL. Inhibitor of fatty acid synthase induced apoptosis in human colonic cancer cells. World J Gastroenterol. 2000;6:295-297. [PubMed] |
| 32. | Li J, Wang WL, Liu B. Angiogenesis and apoptosis in human hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:1057-1060. |
| 33. | Liu HF, Liu WW, Fang DC, Yang SM, Wang RQ. Bax gene ex-pression and its relationship with apoptosis in human gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:665-668. |
| 34. | Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532-539. [PubMed] |
| 35. | Chen HY, Liu WH, Qin SK. Induction of arsenic trioxide on apoptosis of hepatocarcinoma cell lines. Shijie Huaren Xiaohua Zazhi. 2000;8:532-535. |
| 36. | Liu HF, Liu WW, Fang DC. Effect of combined anti-Fas mAb and IFN-γ on the induction of apoptosis in human gastric carci-noma cell line SGC-7901. Shijie Huaren Xiaohua Zazhi. 2000;8:1316-1364. |
| 37. | Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223-227. [PubMed] |
| 38. | Sun ZX, Ma QW, Zhao TD, Wei YL, Wang GS, Li JS. Apoptosis induced by norcantharidin in human tumor cells. World J Gastroenterol. 2000;6:263-265. [PubMed] |
| 39. | Xue XC, Fang GE, Hua JD. Gastric cancer and apoptosis. Shijie Huaren Xiaohua Zazhi. 1999;7:359-361. |
| 40. | Cheng J, Huang HC. Programmed cell death and disease. Peking Union Medical College Press. 1997;1-8. |
| 41. | Brown JM, Wouters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 1999;59:1391-1399. [PubMed] |
| 42. | Ikeda M, Shomori K, Endo K, Makino T, Matsuura T, Ito H. Frequent occurrence of apoptosis is an early event in the oncogenesis of human gastric carcinoma. Virchows Arch. 1998;432:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |