Published online Mar 15, 2003. doi: 10.3748/wjg.v9.i3.622
Revised: July 15, 2002
Accepted: July 22, 2002
Published online: March 15, 2003
AIM: To observe the synthesis of endotoxin receptor CD14 protein and its mRNA expression in Kupffer cells (KCs), and evaluate the role of CD14 in the pathogenesis of liver injury in rats with alcohol-induced liver disease (ALD).
METHODS: Twenty-eight Wistar rats were divided into two groups: ethanol-fed group and control group. Ethanol-fed group was fed ethanol (dose of 5-12g·kg·d-1) and control group received dextrose instead of ethanol. Two groups were sacrificed at 4 wk and 8 wk, respectively. KCs were isolated and the synthesis of CD14 protein and its mRNA expression in KCs were determined by flow cytometric analysis (FCM) or the reverse transcription polymerase chain reaction (RT-PCR) analysis. The levels of plasma endotoxin and alanine transaminase (ALT) were measured by Limulus Amebocyte Lysate assay and standard enzymatic procedures respectively, and the levels of plasma tumor necosis factor (TNF)-α and interleukin (IL)-6 were both determined by ELISA. The liver pathology change was observed under light and electric microscopy.
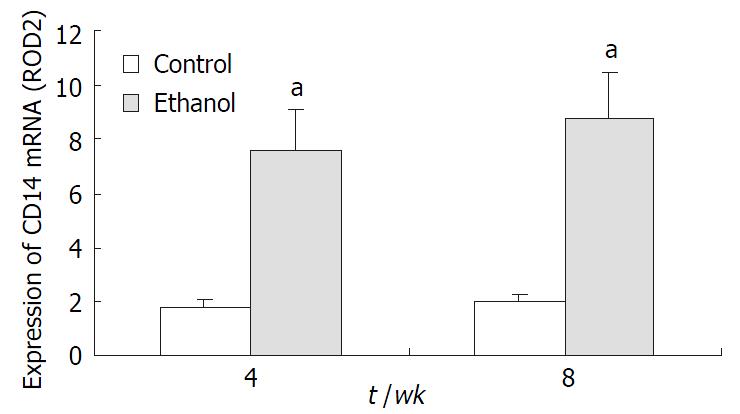
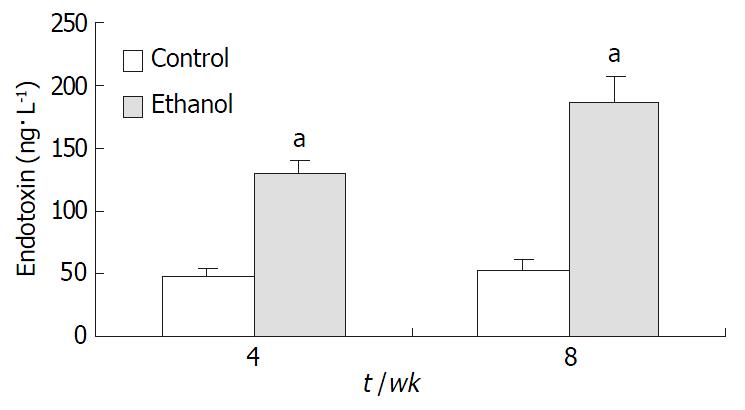
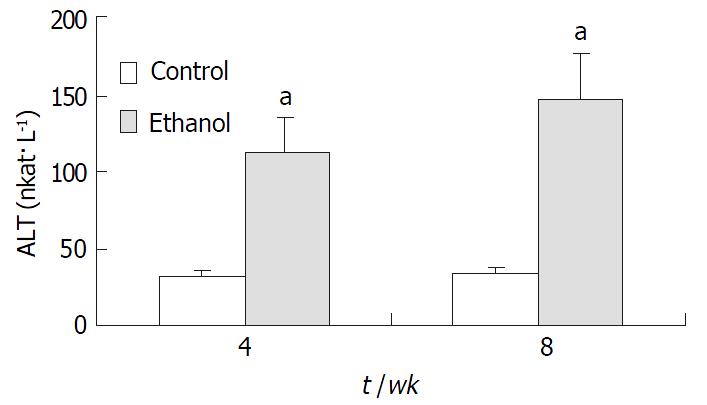
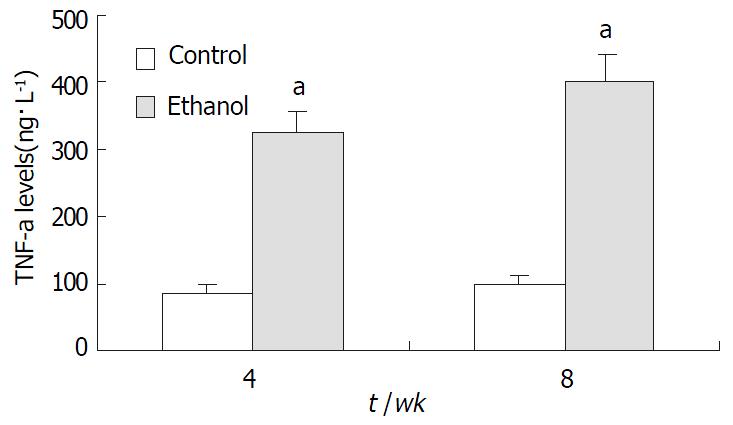
RESULTS: In ethanol-fed group, the percentages of FITC-CD14 positive cells were 76.23% and 89.42% at 4 wk and 8 wk, respectively. Compared with control group (4.45% and 5.38%), the difference was significant (P < 0.05). The expressions of CD14 mRNA were 7.56 ± 1.02 and 8.74 ± 1.37 at 4 wk and 8 wk, respectively, which were significantly higher compared with the control group (1.77 ± 0.21 and 1.98 ± 0.23) (P < 0.05). Plasma endotoxin levels at 4 wk and 8 wk increased significantly in ethanol-fed group (129 ± 21 ng·L-1 and 187 ± 35 ng·L-1) than those in control rats (48 ± 9 ng·L-1 and 53 ± 11 ng·L-1)(P < 0.05). Mean values of plasma ALT levels increased dramatically in ethanol-fed rats (112 ± 15 IU/L and 147 ± 22 IU/L) than those in the control animals (31 ± 12 IU/L and 33 ± 9 IU/L) (P < 0.05). In ethanol-fed rats, the levels of TNF-α were 326 ± 42 ng·L-1 and 402 ± 51 ng·l-1 at 4 wk and 8 wk, respectively which were significantly higher than those in control group (86 ± 12 ng·L-1 and 97 ± 13 ng·L-1) (P < 0.05). The levels of IL-6 were 387 ± 46 ng·L- 1 and 413 ± 51 ng·L-1, which were also higher than control group (78 ± 11 ng·L-1 and 73 ± 10 ng·L-1) (P < 0.05). In liver section from ethanol-fed rats, there were marked pathological changes including steatosis, cell infiltration and necrosis. No marked pathological changes were seen in control group.
CONCLUSION: Ethanol administration led to a significant synthesis of endotoxin receptor CD14 protein and its gene expression in KCs, which maybe result in the pathological changes of liver tissue and hepatic functional damages.
- Citation: Dai LL, Gong JP, Zuo GQ, Wu CX, Shi YJ, Li XH, Peng Y, Deng W, Li SW, Liu CA. Synthesis of endotoxin receptor CD14 protein in Kupffer cells and its role in alcohol-induced liver disease. World J Gastroenterol 2003; 9(3): 622-626
- URL: https://www.wjgnet.com/1007-9327/full/v9/i3/622.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i3.622
Our previous studies have shown that the ethanol-fed rats or rats with endotoxemia had higher endotoxin levels in plasma, increased lipopolysaccharide (LPS) receptor CD14 in the liver, and more serious liver injury compared with control rats[1-6]. But it was unclear where CD14 came from. Accordingly, the purpose of this study is to determine whether Kupffer cells (KCs) synthesize CD14 protein and express its mRNA, and evaluate the role of CD14 protein in alcohol-induced liver disease (ALD).
Twenty-eight adult female Wistar rats weighing 180 g to 220 g were fed ad libitum a liquid diet. Animals were divided into two groups: ethanol liquid diet group (ethanol-fed group) and liquid diet group (control group). The rats of ethanol-fed group were fed ethanol, and control group received the same diet but with isocaloric amounts of dextrose instead of ethanol. In ethanol-fed group, the initial dose is 5 g·kg-1·d-1 and the concentration of ethanol within the diet increased up to 12 g·kg-1·d-1 gradually in 8 weeks. All diets were prepared fresh daily. All the animals were anesthetized with sodium pentobarbital (30 mg·kg-1 intraperitoneally) and sacrificed at 4 wk and 8 wk, respectively. Blood was withdrawn from the portal vein and stored at -70 °C before use.
KCs were isolated with the in situ collagenase perfusion technique, modified as described previously[7,8]. In brief, the livers were removed after a portal vein perfusion with Hanks’ balanced salt solution (HBSS) and the homogenate was digested in a solution of 0.5 g·L-1 collagenase (Type IV, Sigma). The digest was washed thoroughly and plated on plastic dishes in RPMI medium containing 50 mL·L-1 fetal calf serum (FCS). After 3 h incubation at 37 °C in O2 and CO2 (0.95/0.05), nonadherent cells were removed with pipet. The adherent cells were collected with a rubber policeman. KCs purity exceeded 90% as assessed by light microscopy, and viability was typically greater than 92% as determined by trypan blue exclusion assay.
Synthesis of CD14 protein in KCs was examined by FCM[2,3]. In brief, KCs were incubated with the anti-CD14 polyclonal antibody (0.1 mg·L-1) for 30 mins after washing, cells were incubated with goat anti-rabbit immunoglobulin G labled with FITC for 30 min, after being washed for three times, and 10000 cells were analyzed by FCM (Coulter, USA). The percentage and mean fluorescence intensity (MFI) of CD14-positive cells were taken as the indexes.
Total RNA was isolated from KCs with the TRIZOL Reagent (Life Technologies, USA). The quality of RNA was controlled by the intactness of ribosomal RNA bands. 0.5 mg of each intact total RNA samples was reverse-transcribed to complementary DNA (cDNA) with the reverse transcription polymerase chain reaction (RT-PCR) kit (Roche, USA). cDNA was stored at -70 °C until polymerase chain reaction (PCR) analysis.
The PCR primers used were CD14: sense (5’-CTCAACCTAGAGCCGTTTCT-3’), anti-sense (5’-CAGGATTGTCAGACAGGTCT-3’); β-actin: sense (5’-ACCACAGCTGAGAGGGAAATCG-3’), antisense (5’-AGAGGTCTTTACGGATGTCAACG-3’). The sizes of the amplified PCR products were 267 bp for CD14, and 281 bp for β-actin. The reaction conditions for amplification were as below: denaturation at 93 °C for 1 min, annealing at 57 °C for 2 min, and extension at 71 °C for 1 min for 30 cycles. The PCR products were electrophoresed in 20 g·L-1 agarose gels, and the gels were ethidium bromide stained and video photographed on an ultraviolet transilluminator, and the results were showed with the relative absorbance (Ar: relative optical density, ROD).
To determinate the endotoxin, blood was collected into pyrogen-free tubes containing heparin. Plasma was immediately separated at 4 °C by centrifugation at 200 g for 8 minutes and stored in pyrogen-free tubes at -70 °C. Plasma endotoxin levels were measured within a week using the Limulus Amebocyte Lysate assay. Serum alanine transaminase (ALT) was measured by standard enzymatic procedures.
The levels of TNF-α and IL-6 in plasma were determined with enzyme-linked immunosorbent assay (ELISA) kits according to the manufacture’s instructions and guidelines (Biosource International, Camarillo, CA).
Liver samples from left liver lobes were fixed with 100 mL·L-1 buffered formalin or 25 g·L-1 glutaraldehyde immediately. For optical microscope, the tissue blocks were embedded in paraffin, and stained with hematoxylin and eosin (HE). For electronic microscope, the tissue blocks were embedded in Epon 618 resin and ultrathin sections were stained with urany acetate and lead citrate. A H-2000 transmission electron microscope was employed.
All results were expressed as -x±sx. Statistical differences between means were determined using Student’s t test. A P value of ≤ 0.05 was considered significant.
To confirm the synthesis of CD14 protein in KCs, we examined the binding of FITC to the cells. The percentages of FITC-CD14 positive cells at 4 wk and 8 wk were 4.45% and 5.38% in control group, respectively. While in ethanol-fed group, the mean fluorescence intensity (MFI) dramatically increased, the numbers of FITC-CD14 positive cells were 76.23% and 89.42% at 4 wk and 8 wk, respectively. There was significant difference when compared to control group (P < 0.05).
CD14 mRNA expression in both groups KCs was determined with RT-PCR. In control group, there was no significant expression at the level of CD14 mRNA at 4 wk and 8 wk, respectively. However, it was significantly higher in ethanol-fed rats compared with control group (P < 0.05, Figure 1).
Plasma endotoxin levels in ethanol-fed rats increased significantly by ethanol to values of (129 ± 21) ng·L-1 at 4 wk and (187 ± 35) ng·L-1 at 8 wk, the levels of endotoxin were about 2-fold and 3-fold higher than the values of control rats [(48 ± 9) ng·L-1 and (53 ± 11) ng·L-1] (P < 0.05, Figure 2). Mean values for ALT in the control animals were (31 ± 12) nkat·L-1 and (33 ± 9) nkat·L-1 at 4 wk and 8 wk, respectively. Plasma ALT levels were increased dramatically to (112 ± 15) nkat·L-1 and (147 ± 22) nkat·L-1 in ethanol-fed rats after 4 wk and 8 wk, respectively (P < 0.05, Figure 3).
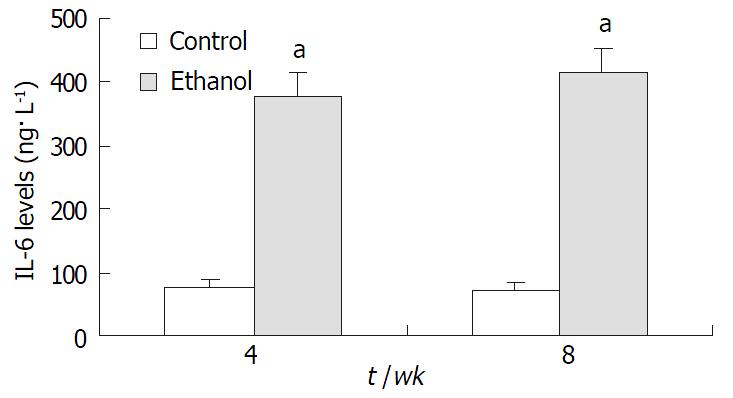
In ethanol-fed group, the plasma concentrations of TNF-α and IL-6 at 4 wk and 8 wk were 326 ± 42 ng•L-1, 402 ± 51 ng•L-1, 387 ± 46 ng•L-1, and 413 ± 51 ng•L-1, respectively, which were higher than those in control group (86 ± 12 ng•L-1, 97 ± 13 ng•L-1, 78 ± 11 ng•L-1, and 73 ± 10 ng•L-1) (P < 0.05, Figure 4 and Figure 5).
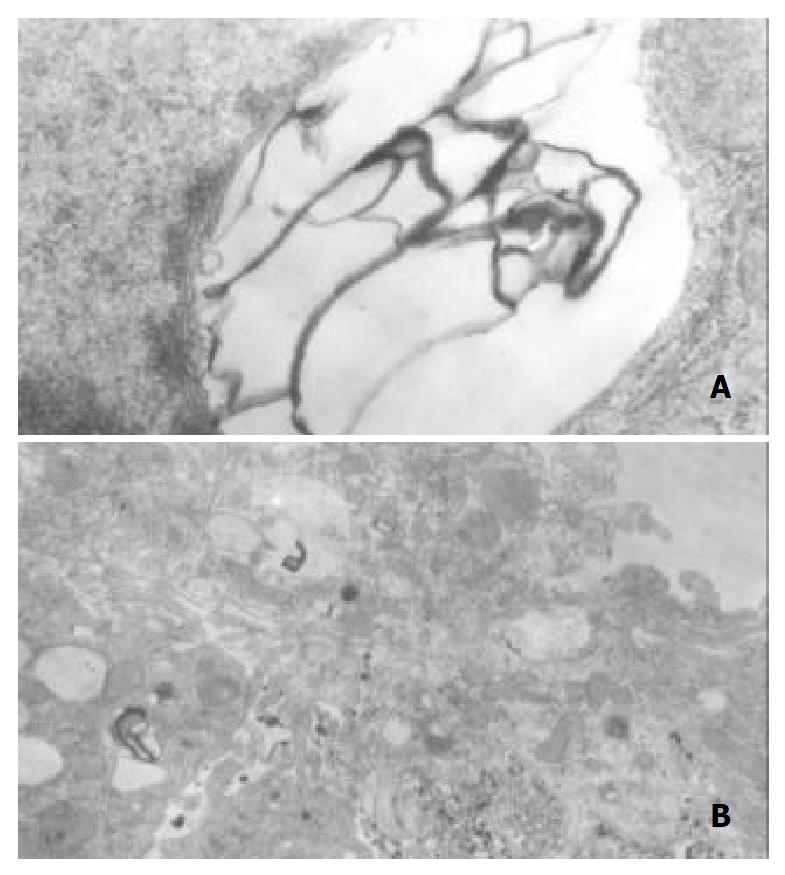
No pathological changes in the liver were observed in the control rats at 4 wk or 8 wk. However, in liver section from rats after 4 wk on ethanol liquid diet, steatosis in both microvesicular and macrovesicular was observed. Few inflammation but accumulation of blood cells in the sinusoidal lining was observed. In liver section from rats after 8 wk on ethanol diet, marked pathological changes (steatosis, cell infiltration and necrosis), KCs proliferation and hypertrophy were detected. Under electron microscopy, huge focal cytoplasmic degeneration and necrosis could be seen in hepatocytes of ethanol-fed rats (Figure 6A). KCs hypertrophy and their surface projections increased as well as, and the phagocytic vacuoles or electron dense phagosomes, Focal cytoplasmic degeneration and many myelin figures in their cytoplasm could also be seen (Figure 6B).
Many studies have documented that liver disease could result from the dose- and time-dependent consumption of alcohol[1,9,10]. However, the mechanisms remain unclear. There appears to be increasing evidences that ethanol toxicity maybe associated with increased levels of endotoxin in plasma[9,11-16]. Endotoxin or LPS is believed to exert many of its effects on the liver injury via interacting with LBP and CD14[17-23]. LBP and CD14 are clearly implicated in the molecular and cellular basis of the interaction between endotoxin and monocytes/macrophages. LBP in serum can recognize and bind LPS to form LPS-LBP complexes and activate cells through CD14 receptor on the membrane of these cells, initiate a process leading to the release of cytokines (e.g., tumor necrosis factor-α and interleukines), prostanoids, and other soluble mediators[9,10,24-29]. The release of these mediators is considered to be an early key step in the pathogenesis of liver disease because they trigger inflammatory events in the liver and alter the parenchymal homeostasis, ultimately initiate liver injury[30-34]. But, it is not clear where CD14 in liver comes from.
The major goal of this study is to observe the synthesis of CD14 protein and its mRNA expression in KCs in ethanol-fed rats and evaluate the its role in ALD. It was found that endotoxin levels in the plasma of rats treated with ethanol were increased significantly when compared with control animals. The increase in CD14 mRNA levels in the ethanol-fed rats is correlated to inflammation degree and necrosis in the liver of these animals, which developed fatty liver, necrosis, and inflammation. Control rats showed no liver pathological changes. In the present study, we found the severity of pathological changes in ethanol-fed rats was accompanied with the increase in CD14 mRNA in KCs and serum ALT. A similar pattern of changes was observed by Yin et al[12]. They found that blood endotoxin and hepatic CD14 mRNA and protein were increased by ethanol. Therefore, the sensitivity (vulnerability) of rat liver to alcohol-induced injury is directly related to CD14 expression in the liver, leading to the increased production of TNF-α, free radicals, interleukins and other cytokines[35-39]. The marked increase in the synthesis of CD14 protein suggests a new mechanism by which alcohol enhanced the LPS-mediated cytokine signaling by the liver macrophages, thus promoting the interaction between alcohol and endotoxins in the development of liver injury[40-43].
It has been well established that the Liver is the main source of acute reaction protein[1-6,8]. However, the mechanism by which the synthesis of CD14 protein and its mRNA expression in the liver increased is thus as yet unclear. It is reported that the synthesis of CD14 protein and its gene expression occurred mainly in monocytes/macrophages, including the cells that reside in the liver (KCs) and these may represent recruitment of inflammatory cells, for example, infiltrating mononuclear cells or macrophages that have high expression of CD14 gene and CD14 protein[44-48]. The increased CD14 protein may promote monocytes/macrophages response to low level of endotoxin, and lead to NF-kB activation and production of pro-inflammatory cytokines, which play an important role in alcoholic liver injury[49-53].
In summary, our results show that ethanol administration led to a significant increase of CD14 protein synthesis and its mRNA expression in KCs when compared with the control rats. The synthesis of CD14 protein and its gene expression may result in greater sensitivity of hepatocytes to endotoxin and lead to the pathological changes of liver tissue and liver functional injury.
Edited by Bo XN
| 1. | Zuo GQ, Gong JP, Liu CA, Li SW, Wu XC, Yang K, Li Y. Expression of lipopolysaccharide binding protein and its receptor CD14 in experimental alcoholic liver disease. World J Gastroenterol. 2001;7:836-840. [PubMed] |
| 2. | Li SW, Gong JP, Wu CX, Shi YJ, Liu CA. Lipopolysaccharide induced synthesis of CD14 proteins and its gene expression in hepatocytes during endotoxemia. World J Gastroenterol. 2002;8:124-127. [PubMed] |
| 3. | Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961-965. [PubMed] |
| 4. | Li S, Wu C, Shi Y, Liu C. [Lipopolysaccharide upregulates expression of CD14 gene and CD14 proteins of hepatocytes in rats]. Zhonghua Ganzangbing Zazhi. 2001;9:103-104. [PubMed] |
| 5. | Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Li XH, Peng Y. Liver sinusoidal endothelial cell injury by neutrophils in rats with acute obstructive cholangitis. World J Gastroenterol. 2002;8:342-345. [PubMed] |
| 6. | Gong JP, Han BL. Role of CD14 in activation of Kupffer cell in-duced by lipopolysaccharide. Shijie Huaren Xiaohua Zazhi. 1999;7:875-877. |
| 7. | Gong JP, Han BL. Isolation, culture and identification of liver cells. S. hijie Huaren Xiaohua Zazhi. 1999;7:417-419. |
| 8. | Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Yang K, Li Y, Li XH. Intestinal damage mediated by Kupffer cells in rats with endotoxemia. World J Gastroenterol. 2002;8:923-927. [PubMed] |
| 9. | Järveläinen HA, Fang C, Ingelman-Sundberg M, Lindros KO. Effect of chronic coadministration of endotoxin and ethanol on rat liver pathology and proinflammatory and anti-inflammatory cytokines. Hepatology. 1999;29:1503-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Lin H, Lu M, Zhang YX, Wang BY, Fu BY. Induction of a rat model of alcoholic liver disease. Shijie Huaren Xiaohua Zazhi. 2001;9:24-28. |
| 12. | Yin M, Ikejima K, Wheeler MD, Bradford BU, Seabra V, Forman DT, Sato N, Thurman RG. Estrogen is involved in early alcohol-induced liver injury in a rat enteral feeding model. Hepatology. 2000;31:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 381] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 14. | French SW. Intragastric ethanol infusion model for cellular and molecular studies of alcoholic liver disease. J Biomed Sci. 2001;8:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Enomoto N, Ikejima K, Yamashina S, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Schemmer P, Bradford BU, Rivera CA. Kupffer cell-derived prostaglandin E(2) is involved in alcohol-induced fat accumulation in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G100-G106. [PubMed] |
| 16. | Bautista AP. Impact of alcohol on the ability of Kupffer cells to produce chemokines and its role in alcoholic liver disease. J Gastroenterol Hepatol. 2000;15:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Adhesion molecule and proinflammatory cytokine gene expression in hepatic sinusoidal endothelial cells following cecal ligation and puncture. World J Gastroenterol. 2001;7:128-130. [PubMed] |
| 18. | Ling YL, Meng AH, Zhao XY, Shan BE, Zhang JL, Zhang XP. Effect of cholecystokinin on cytokines during endotoxic shock in rats. World J Gastroenterol. 2001;7:667-671. [PubMed] |
| 19. | Hiki N, Berger D, Mimura Y, Frick J, Dentener MA, Buurman WA, Seidelmann M, Kaminishi M, Beger HG. Release of endotoxin-binding proteins during major elective surgery: role of soluble CD14 in phagocytic activation. World J Surg. 2000;24:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Gutsmann T, Müller M, Carroll SF, MacKenzie RC, Wiese A, Seydel U. Dual role of lipopolysaccharide (LPS)-binding protein in neutralization of LPS and enhancement of LPS-induced activation of mononuclear cells. Infect Immun. 2001;69:6942-6950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Heumann D, Adachi Y, Le Roy D, Ohno N, Yadomae T, Glauser MP, Calandra T. Role of plasma, lipopolysaccharide-binding protein, and CD14 in response of mouse peritoneal exudate macrophages to endotoxin. Infect Immun. 2001;69:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Scott MG, Vreugdenhil AC, Buurman WA, Hancock RE, Gold MR. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol. 2000;164:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 212] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, Bradford BU, Forman DT, Thurman RG. Gender differences in early alcohol-induced liver injury: role of CD14, NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2000;278:G652-G661. [PubMed] |
| 24. | Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 397] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | Wang LS, Zhu HM, Zhou DY, Wang YL, Zhang WD. Influence of whole peptidoglycan of bifidobacterium on cytotoxic effectors produced by mouse peritoneal macrophages. World J Gastroenterol. 2001;7:440-443. [PubMed] |
| 26. | Bai XY, Jia XH, Cheng LZ, Gu YD. Influence of IFN alpha-2b and BCG on the release of TNF and IL-1 by Kupffer cells in rats with hepatoma. World J Gastroenterol. 2001;7:419-421. [PubMed] |
| 27. | Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1197] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 28. | McCaughan GW, Gorrell MD, Bishop GA, Abbott CA, Shackel NA, McGuinness PH, Levy MT, Sharland AF, Bowen DG, Yu D. Molecular pathogenesis of liver disease: an approach to hepatic inflammation, cirrhosis and liver transplant tolerance. Immunol Rev. 2000;174:172-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Hedin KE, Kaczynski JA, Gibson MR, Urrutia R. Transcription factors in cell biology, surgery, and transplantation. Surgery. 2000;128:1-5. [PubMed] |
| 30. | Bone-Larson CL, Simpson KJ, Colletti LM, Lukacs NW, Chen SC, Lira S, Kunkel SL, Hogaboam CM. The role of chemokines in the immunopathology of the liver. Immunol Rev. 2000;177:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Martin M, Katz J, Vogel SN, Michalek SM. Differential induction of endotoxin tolerance by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. J Immunol. 2001;167:5278-5285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Enomoto N, Yamashina S, Kono H, Schemmer P, Rivera CA, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Thurman RG. Development of a new, simple rat model of early alcohol-induced liver injury based on sensitization of Kupffer cells. Hepatology. 1999;29:1680-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Ikejima K, Enomoto N, Seabra V, Ikejima A, Brenner DA, Thurman RG. Pronase destroys the lipopolysaccharide receptor CD14 on Kupffer cells. Am J Physiol Gastrointest Liver Physiol. 1999;276:G591-G598. |
| 34. | Järveläinen HA, Orpana A, Perola M, Savolainen VT, Karhunen PJ, Lindros KO. Promoter polymorphism of the CD14 endotoxin receptor gene as a risk factor for alcoholic liver disease. Hepatology. 2001;33:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Shoham S, Huang C, Chen JM, Golenbock DT, Levitz SM. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. J Immunol. 2001;166:4620-4626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Guo X, Dudman NP. Homocysteine induces expressions of adhesive molecules on leukocytes in whole blood. Chin Med J (Engl). 2001;114:1235-1239. [PubMed] |
| 38. | LeVan TD, Bloom JW, Bailey TJ, Karp CL, Halonen M, Martinez FD, Vercelli D. A common single nucleotide polymorphism in the CD14 promoter decreases the affinity of Sp protein binding and enhances transcriptional activity. J Immunol. 2001;167:5838-5844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 257] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 39. | Nanbo A, Nishimura H, Muta T, Nagasawa S. Lipopolysaccharide stimulates HepG2 human hepatoma cells in the presence of lipopolysaccharide-binding protein via CD14. Eur J Biochem. 1999;260:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Gordon H. Detection of alcoholic liver disease. World J Gastroenterol. 2001;7:297-302. [PubMed] |
| 41. | MacDonald GA, Bridle KR, Ward PJ, Walker NI, Houglum K, George DK, Smith JL, Powell LW, Crawford DH, Ramm GA. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J Gastroenterol Hepatol. 2001;16:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Calne RY. Immunological tolerance--the liver effect. Immunol Rev. 2000;174:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 395] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 44. | Heinrich JM, Bernheiden M, Minigo G, Yang KK, Schütt C, Männel DN, Jack RS. The essential role of lipopolysaccharide-binding protein in protection of mice against a peritoneal Salmonella infection involves the rapid induction of an inflammatory response. J Immunol. 2001;167:1624-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Neilsen PO, Zimmerman GA, McIntyre TM. Escherichia coli Braun lipoprotein induces a lipopolysaccharide-like endotoxic response from primary human endothelial cells. J Immunol. 2001;167:5231-5239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Jersmann HP, Hii CS, Hodge GL, Ferrante A. Synthesis and surface expression of CD14 by human endothelial cells. Infect Immun. 2001;69:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Funda DP, Tucková L, Farré MA, Iwase T, Moro I, Tlaskalová-Hogenová H. CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited. Infect Immun. 2001;69:3772-3781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Le Roy D, Di Padova F, Adachi Y, Glauser MP, Calandra T, Heumann D. Critical role of lipopolysaccharide-binding protein and CD14 in immune responses against gram-negative bacteria. J Immunol. 2001;167:2759-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001;166:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 295] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 50. | Gong JP, Liu CA, Wu CX, Li SW, Shi YJ, Li XH. Nuclear factor kB activity in patients with acute severe cholangitis. World J Gastroenterol. 2002;8:346-349. [PubMed] |
| 51. | Medvedev AE, Henneke P, Schromm A, Lien E, Ingalls R, Fenton MJ, Golenbock DT, Vogel SN. Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4. J Immunol. 2001;167:2257-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165:3541-3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 251] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 53. | Järveläinen HA, Fang C, Ingelman-Sundberg M, Lukkari TA, Sippel H, Lindros KO. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. J Hepatol. 2000;32:900-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |