Published online Mar 15, 2003. doi: 10.3748/wjg.v9.i3.491
Revised: December 23, 2002
Accepted: January 9, 2003
Published online: March 15, 2003
AIM: To investigate the expression and significance of PTEN, hypoxia-inducible factor-1 alpha (HIF-1α), and targeting gene VEGF during colorectal carciogenesis.
METHODS: Total 71 cases colorectal neoplasms (9 cases of colorectal adenoma and 62 colorectal adenocarcinoma) were formalin fixed and paraffin-embedded, and all specimens were evaluated for PTEN mRNA, HIF-1α mRNA and VEGF protein expression. PTEN mRNA, HIF-1α mRNA were detected by in situ hybridization. VEGF protein was identified by citrate-microwave SP immunohistochemical method.
RESULTS: There were significant differences in PTEN, HIF-1α and VEGF expression between colorectal adenomas and colorectal adenocarcinoma (P < 0.05). The level of PTEN expression decreased as the pathologic stage increased. Conversely, HIF-1α and VEGF expression increased with the Dukes stage as follows: stage A (0.1029 ± 0.0457: 0.1207 ± 0.0436), stage B (0.1656 ± 0.0329: 0.1572 ± 0.0514), and stage C + D (0.2335 ± 0.0748: 0.2219 ± 0.0803). For PTEN expression, there was a significant difference among Dukes stage A, B, and C + D, and the level of PTEN expression was found to be significant higher in Dukes stage A or B than that of Dukes stage C or D. For HIF-1α expression, there was a significant difference between Dukes stage A and B, and the level of HIF-1α expression was found to be significantly higher in Dukes stage C+D than that of Dukes stage A or B. The VEGF expression had similar results as HIF-1α expression. In colorectal adenocarcinoma, decreased levels of PTEN were significantly associated with increased expression of HIF-1α mRNA (r = -0.36, P < 0.05) and VEGF protein (r = -0.48, P < 0.05) respectively. The levels of HIF-1 were positively correlated with VEGF expression (r = 0.71, P < 0.01).
CONCLUSION: Loss of PTEN expression and increased levels of HIF-1α and VEGF may play an important role in carcinogenesis and progression of colorectal adenocarcinoma.
- Citation: Jiang YA, Fan LF, Jiang CQ, Zhang YY, Luo HS, Tang ZJ, Xia D, Wang M. Expression and significance of PTEN, hypoxia-inducible factor-1 alpha in colorectal adenoma and adenocarcinoma. World J Gastroenterol 2003; 9(3): 491-494
- URL: https://www.wjgnet.com/1007-9327/full/v9/i3/491.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i3.491
So far, the mechanism of colorectal oncogenesis is not fully understood. Recent studies[1-6] have reported on the association of a tumor suppressor gene PTEN with the oncogeneses of several type cancer and cancer cell lines and on PTEN playing an important role in the tumor progression and metastases. Moreover, hypoxia-inducible Factor-1 alpha (HIF-1α) is a transcription factor identified as being activated by hypoxia, and plays a central role in tumor angiogenesis[7-19]. Therefore, this study was undertaken to investigate the expression and relationship between PTEN and HIF-1α and VEGF in colorectal carcinogenesis.
The specimens of colorectal cancer were surgically obtained from 71 patients at the Hospital of Wuhan University between 1996 and 1997, and 71 patients were catalogued by histological subtypes as follows: 62 cases of colorectal adenocarcinoma (17 cases in Dukes stage A, 18 stage B, 20 stage C and 7 stage D) and 9 patients of colorectal adenoma (5 cases of tulubo-villous adenoma, 2 tubular adenoma and 2 villous adenoma).
A rabbit VEGF polyclonal antibody was purchased from Neomark International Corporation (Taipei, Taiwan), PTEN mRNA, HIF - 1 α mRNA in situ hy b rid izatio n an d immunohistochemical repairase were purchased from Boster Corporation ( Wuhan ,China), and S-P reagent was purchased from Maixin Corporation (Fuzhou, China).
Sections were dewaxed in xylene, taken thorough ethanol, and then incubated with 3% hydrogen peroxide to block endogenous peroxidase activity. Sections then were repaired by repairase for 10 minutes, and the procedure of immunohistochemical determination was performed according to the manufacturer’s instruction. A rabbit polyclonal antibody was a dilution of 1:50.
Frozen sections were cut onto slides, briefly air-dried, and stored at -80 °C. Prior to use they were fixed in 4% paraformaldehyde in PBS for 20 min, washed in PBS, and treated with proteinase K (0.0005%) in 0.1 M Tris/0.05 M EDTA, pH 8.0 for 5 min at 37 °C. Slides were rinsed in 0.2% glycine in water, postfixed in 4% paraformaldehyde/0.1 M NaOH/0.1 M NaAc for 5 min, rinsed in 0.1 M triethanolamine (TEA), pH 8.0 for 3 min, acetylated for 10 min in 0.25% acetic anhydride/0.1 M TEA, pH 8.8, washed in 2 × SSC, and dehydrated. RNA probe was then hybridized to the sections at 60 °C for 16 hrs in 50% formamide/10% dextran sulfate/0.15 M NaCl/1 × Denhardt’s solution/0.01 M Tris·Cl, pH 8.0/0.01 M DTT with 0.5 mg/ml tRNA. Sections from each tumor were always hybridized to sense probes as a control for specificity. The slides were next rinsed in 4 × SSC and incubated at 37 °C for 30 min with 0.1 mg/ml RNaseA in 0.5 M NaCl/0.01 M Tris·Cl, pH 8.0/1 mM EDTA. They were then desalted, dehydrated through graded ethanols, and coated with emulsion. Following exposure at 4 °C for 5 days, emulsion was developed and fixed, and sections were stained with hematoxylin and eosin. To analyse image scanning, we obtained value of absorbance.
All results were expressed as the means ± SD. Statistical analyses, including the Chi-square test and correlated Spearman test, were carried out with the software package SPSS10.0. A P value of < 0.05 was considered statistically significant.
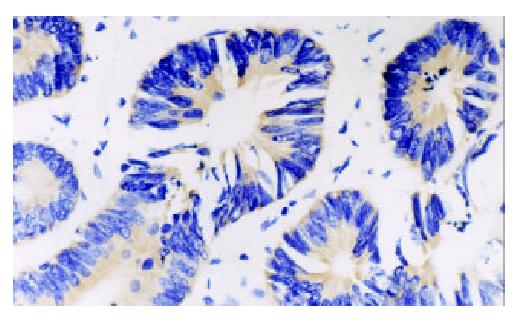
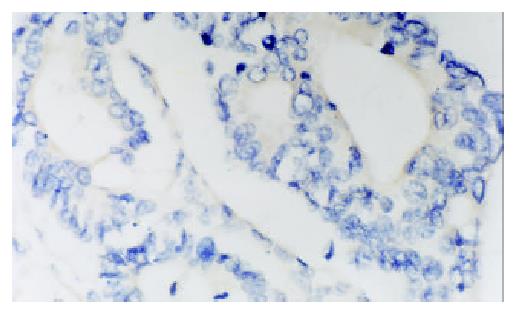
PTEN mRNA and HIF-1α mRNA expression was brown granular, and localized in cytoplasm of tumor cells (Figures 1 and 2). HIF-1α mRNA expression was mainly localized in cytoplasm of tumor cells, frequently observed in margin of tumor necrotic zones. VEGF expression was mainly localized in cytoplasm of tumor cells, also observed in endothelial cell of blood vessel (Figure 3). Expression of PTEN mRNA, HIF-1α mRNA and VEGF protein was detected in 7, 4 and 3 cases of 9 colorectal adenomas respectively. PTEN mRNA expression was significantly higher (P < 0.05) in adenomas than that in adenocarcinoma, but HIF-1α mRNA expression was significantly lower (P < 0.05) in adenomas than that in adenocarcinoma. There was a significant difference (P < 0.05) in VEGF expression between colorectal adenomas and adenocarcinoma.
Table 1 shows the correlation between PENT, HIF-1α,VEGF expression and Dukes stages of colorectal adenocarcinoma. For PTEN expression, there was a significant difference among Dukes stage A, B, C and D, and the level of PTEN expression was found to be significant higher in Dukes stage A or B than that in Dukes stage C + D. For HIF-1α expression, there was a significant difference between Dukes stage A and Dukes stage B, and the level of HIF-1α expression was found to be significantly higher in Dukes stage C + D than that in Dukes stage A or B. VEGF expression had same results as HIF-1α expression.
The Spearman analysis showed that the level of PTEN was significantly associated with HIF-1α expression (r = -0.36, P < 0.05) and with VEGF protein expression (r = -0.68, P < 0. 05) respectively. The level of HIF-1α was correlated with VEGF expression (r = 0.72, P < 0.01).
PTEN (phosphatase and Tensin homologue deleted on chromosome 10) or MMAC1 (mutated in multiple advanced cancers 1) was recently reported to undergo frequent genetic alterations, including mutations and deletions in multiple advanced cancers[20-22]. PTEN located at chromosome 10q23.3 encodes a dual-specificity phosphatase that negatively regulates the phosphoinositol- 3-kinase(PI3K)/Akt (protein kinase B) pathway and mediates cell cycle arrest and apotosis[23]. PTEN protein contains sequence motifs with significant homology to the catalytic domain of protein phosphatases and to the cytoskeletal protein, tensin, and auxilin[24]. PTEN mutations and deletions were observed in a number of glioma, prostate, kidney and breast carcinoma cell lines or tumor specimens[25-27]. Rencently, Shin et al[28] screened the PTEN gene in 32 colorectal cancers (8 cell lines and 24 tissues), displaying microsatellite instability (MSI) and six frameshift mutations. We observed that PTEN mRNA decreased as the pathological stage increased, and was significantly associated with VEGF protein expression (r = -0.68, P < 0.05) in colorectal adenoma and adenocarcinoma. These findings suggested that PTEN alteration was possibly involved in the tumor progression and formation of metastasis, and the roles of PTEN in tumor progression and metastasis may be correlated with VEGF expression. Hwang et al[29] observed that PTEN inhibited the tumorigenicity of B16F10 melanoma cells, and their results suggested that PTEN inhibited tumorigenicity and metastasis through regulating VEGF expression. Jiang et al[30] found that the overexpression of PTEN inhibited angiogenesis in chicken embryos, and that the PTEN overexpression inhibited the VEGF expression through the PI 3-kinase or Akt dependent pathway. Our results also indicated that PTEN played an important role by inhibiting VEGF expression in colorectal oncogenesis.
HIF-1 is a BHLH-PAS transcription factor that plays an essential role in O2 homeostasis[31-34]. HIF-1 is a heterodimer composed of HIF-1α and HIF-1β subunits[31]. HIF-1β (also known as the aryl hydrocarbon receptor nuclear translocator) is a common subunit of multiple BHLH-PAS proteins, whereas HIF-1α is the unique, O2-regulated subunit that determines HIF-1 activity[35,36]. HIF-1α activates the transcription of genes encoding transferrin, VEGF, endothelin-1 and inducible nitric oxide synthase, which are implicated in vasodilation, neovascularization, and tumor metastasis[36,37]. Many studies had identified that HIF-1α protein was overexpressed in multiple types of human cancer including lung, prostate, breast, and colon carcinomas, even in preneoplastic and premalignant lesions[38-42]. More importantly, Birner et al[43] found that the overexpression of HIF-1α is an important marker in precancerous lesion such as early-stage cervical cancer, cervical intra-epithelial neoplasia III, early stage lymph node-negative breast cancer. We also found that the levels of HIF-1α mRNA increased gradually as the pathologic stage increased, and were statistically significantly associated with VEGF expression. The same alterations were observed in other tumor tissues. Our findings further identified that HIF-1α and VEGF played an important role in the tumor angiogeneses and formations of metastases. In this study, we found that HIF-1α mRNA and VEGF were overexpressed in 4 and 3 cases of colorectal adenomas respectively, and suggested that cell hypoxia occurred prior to carciogenesis, and persisted to subsequent progression. Generally, these data suggested that HIF-1α overexpression may be an early stage of carciogenesis and it occurred prior to angiogenesis or invasion which morphologically confirmed. In this study, the expression of HIF-1α mRNA and VEGF was significantly higher in the tissues of Dukes stage C/D of colorectal adenocarcinoma than those in Dukes stage A/B, indicating that HIF was involved in the tumor invasion and formation of metastasis. Thus, we believe that the HIF-1α mRNA and VEGF overexpression is a strong independent prognostic marker in colorectal tumor.
That HIF-1 expression was activated and regulated by EGFR, HER2, and IGFR through PI3K/Akt/ FRAP (FKBP-rapamycin-associated protein) pathway had been identified, and a tumor suppressor gene PTEN regulated the HIF-1α expression and transcription activation by inhibiting PI3K/Akt/ FRAP pathway[44,45]. The loss of wild-type PTEN resulted in HIF-1α overexpression, and contributed to the formations of tumor angiogeneses in human prostate cancer and glioma. Our findings that the levels of HIF-1α were negatively correlated with VEGF expression and the level of PTEN were positively correlated with VEGF expression had further identified that loss of PTEN expression and increased levels of HIF-1α and VEGF may play an important role in carcinogenesis and progression of colorectal carcinoma.
Edited by Zhang JZ
| 1. | Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen KL, Vinson VL, Gumpper KL. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997;57:5221-5225. [PubMed] |
| 2. | Tsuchiya KD, Wiesner G, Cassidy SB, Limwongse C, Boyle JT, Schwartz S. Deletion 10q23.2-q23.33 in a patient with gastrointestinal juvenile polyposis and other features of a Cowden-like syndrome. Genes Chromosomes Cancer. 1998;21:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Tsao H, Zhang X, Benoit E, Haluska FG. Identification of PTEN/MMAC1 alterations in uncultured melanomas and melanoma cell lines. Oncogene. 1998;16:3397-3402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Yang L, Kuang LG, Zheng HC, Li JY, Wu DY, Zhang SM, Xin Y. PTEN encoding product: a marker for tumorigenesis and progression of gastric carcinoma. World J Gastroenterol. 2003;9:35-39. [PubMed] |
| 5. | Wen S, Stolarov J, Myers MP, Su JD, Wigler MH, Tonks NK, Durden DL. PTEN controls tumor-induced angiogenesis. Proc Natl Acad Sci U S A. 2001;98:4622-4627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Poetsch M, Dittberner T, Woenckhaus C. PTEN/MMAC1 in malignant melanoma and its importance for tumor progression. Cancer Genet Cytogenet. 2001;125:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int. 1997;51:553-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 242] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1997;94:8104-8109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 793] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 9. | Minet E, Michel G, Remacle J, Michiels C. Role of HIF-1 as a transcription factor involved in embryonic development, cancer progression and apoptosis (review). Int J Mol Med. 2000;5:253-259. [PubMed] |
| 10. | Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Søndergaard KL, Hilton DA, Penney M, Ollerenshaw M, Demaine AG. Expression of hypoxia-inducible factor 1alpha in tumours of patients with glioblastoma. Neuropathol Appl Neurobiol. 2002;28:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 959] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 13. | Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst. 2001;93:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 450] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | Zhong H, Hanrahan C, van der Poel H, Simons JW. Hypoxia-inducible factor 1alpha and 1beta proteins share common signaling pathways in human prostate cancer cells. Biochem Biophys Res Commun. 2001;284:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Nakayama K, Kanzaki A, Hata K, Katabuchi H, Okamura H, Miyazaki K, Fukumoto M, Takebayashi Y. Hypoxia-inducible factor 1 alpha (HIF-1 alpha) gene expression in human ovarian carcinoma. Cancer Lett. 2002;176:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Semenza GL. Involvement of hypoxia-inducible factor 1 in human cancer. Intern Med. 2002;41:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Koukourakis MI, Giatromanolaki A, Sivridis E, Simopoulos C, Turley H, Talks K, Gatter KC, Harris AL. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:1192-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 262] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010-4015. [PubMed] |
| 19. | Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 781] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 20. | Chang JG, Chen YJ, Perng LI, Wang NM, Kao MC, Yang TY, Chang CP, Tsai CH. Mutation analysis of the PTEN/MMAC1 gene in cancers of the digestive tract. Eur J Cancer. 1999;35:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Guanti G, Resta N, Simone C, Cariola F, Demma I, Fiorente P, Gentile M. Involvement of PTEN mutations in the genetic pathways of colorectal cancerogenesis. Hum Mol Genet. 2000;9:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2036] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 23. | Zhou XP, Loukola A, Salovaara R, Nystrom-Lahti M, Peltomäki P, de la Chapelle A, Aaltonen LA, Eng C. PTEN mutational spectra, expression levels, and subcellular localization in microsatellite stable and unstable colorectal cancers. Am J Pathol. 2002;161:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 146] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2036] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 25. | Koul D, Shen R, Garyali A, Ke LD, Liu TJ, Yung WK. MMAC/PTEN tumor suppressor gene regulates vascular endothelial growth factor-mediated angiogenesis in prostate cancer. Int J Oncol. 2002;21:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Velickovic M, Delahunt B, McIver B, Grebe SK. Intragenic PTEN/MMAC1 loss of heterozygosity in conventional (clear-cell) renal cell carcinoma is associated with poor patient prognosis. Mod Pathol. 2002;15:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Harima Y, Sawada S, Nagata K, Sougawa M, Ostapenko V, Ohnishi T. Mutation of the PTEN gene in advanced cervical cancer correlated with tumor progression and poor outcome after radiotherapy. Int J Oncol. 2001;18:493-497. [PubMed] |
| 28. | Shin KH, Park YJ, Park JG. PTEN gene mutations in colorectal cancers displaying microsatellite instability. Cancer Lett. 2001;174:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Hwang PH, Yi HK, Kim DS, Nam SY, Kim JS, Lee DY. Suppression of tumorigenicity and metastasis in B16F10 cells by PTEN/MMAC1/TEP1 gene. Cancer Lett. 2001;172:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000;97:1749-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 421] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 31. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4392] [Cited by in RCA: 4718] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 32. | Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1963] [Cited by in RCA: 1947] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 33. | Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005-3015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1190] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 34. | Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1877] [Cited by in RCA: 1951] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 35. | Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172-C1180. [PubMed] |
| 36. | Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1475] [Cited by in RCA: 1486] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 37. | Kerbel RS. New targets, drugs, and approaches for the treatment of cancer: an overview. Cancer Metastasis Rev. 1998;17:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881-890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 371] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 39. | Saramäki OR, Savinainen KJ, Nupponen NN, Bratt O, Visakorpi T. Amplification of hypoxia-inducible factor 1alpha gene in prostate cancer. Cancer Genet Cytogenet. 2001;128:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res. 2002;8:1831-1837. [PubMed] |
| 41. | Welsh SJ, Bellamy WT, Briehl MM, Powis G. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 2002;62:5089-5095. [PubMed] |
| 42. | Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830-5835. [PubMed] |
| 43. | Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693-4696. [PubMed] |
| 44. | Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995-4004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 966] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 45. | Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541-1545. [PubMed] |