Published online Mar 15, 2003. doi: 10.3748/wjg.v9.i3.485
Revised: July 13, 2002
Accepted: July 27, 2002
Published online: March 15, 2003
AIM: To investigate the photodynamic inhibitory effects of Elsinochrome A (EA), Hypocrellin A (HA) and Hypocrellin B (HB) on human colorectal carcinoma Hce-8693 cells and rhesus monkey embryonic stem R366.4 cells, via inducing apoptosis.
METHODS: EA, HA and HB were extracted from metabolites of Hypomyces (Fr) Tul.Sp. R366.4 cells or Hce-8693 cells were cultured with different concentrations of EA, HA or HB respectively, irradiated and incubated with fresh medium for 2 h. Cell cycle analysis was performed by flow cytometry (FCM). Data were expressed as means ± SD and analysis of variance and Student’t-test for individual comparisons.
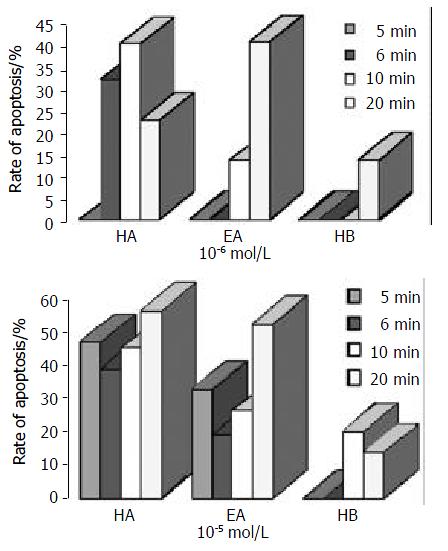
RESULTS: The photodynamic bioactivity of EA was first reported in this study. After irradiation for 5 min, 6 min, 10 min or 20 min, photoactivated EA at lower concentrations, which were 10-7 Mol/L, 10- 6 Mol/L, 10 - 5 Mol/L respectively, had no cytotoxic effects on R366.4 ES cells. Whereas, all of the three perylenequinones could induce apoptosis with a dose-dependent manner when Hce-8693 cells were incubated with photoactivated EA, HA and HB respectively. When Hce-8693 cells were incubated with EA at 10-6 Mol/L and irradiated 5 min, 6 min, 10 min and 20 min respectively, the rates of EA-induced apoptosis were 0, 0, 13.4% and 40.5%. While the rates of HA-induced apoptosis were 29.5%, 32.0%, 40.2% and 22.6%. And the rates of HB-induced apoptosis were 0, 0, 0 and 13.7% respectively. Meanwhile, after 10-5 Mol/L treatment, the rates of EA-induced apoptosis were 32.7%, 19.3%, 26.4% and 52.7%, the rates of HA-induced apoptosis were 47.2%, 39.1%, 45.2% and 56.6%, and the rates of HB-induced apoptosis were 0, 0, 20.0% and 13.9% respectively.
CONCLUSION: EA, HA and HB have significant anti-cancer activity. The order of photodynamic inhibitory effects on tumor cells would be approximately HA > EA > HB. The molecular mechanisms of apoptosis may not be induced by reactive oxygen species and are worth further investigation.
- Citation: Ma L, Hong T, Li C, Zhang Y, Wang ZH, Ji WZ. Photodynamic inhibitory effects of three perylenequinones on human colorectal carcinoma cell line and primate embryonic stem cell line. World J Gastroenterol 2003; 9(3): 485-490
- URL: https://www.wjgnet.com/1007-9327/full/v9/i3/485.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i3.485
Perylenequinones are a type of photosensitive pigments widespread in nature, which have been isolated from fungi, as well as other organisms[1-5]. These lipid-soluble 4.9-dihydroxy-3, 10-perylenequinone derivatives are efficient producers of singlet oxygen (1O2) in visible light[6-11]. Due to their excellent photosensitive properties, they are expected to be developed as new phototherapeutic medicines[8,12-17]. Among them, Elsinochrome A (EA) was first reported in 1966 by Chen CT et al who isolated EA from Elsinoe spp. I[1-2]. And Meille SV et al reported the structure of EA[18]. Since then, there are no more related reports about EA. Hypocrellins are well-known photosensitizers, including hypocrellin A (HA) and hypocrellin B (HB), isolated from natural fungus sacs of Hypocrella bambusae growing in north western region of Yunnan Province in China[19]. Hypocrellins were potent inhibitors of protein kinase C (PKC)[20], and could inactivate some types of viruses in the presence of visible light and oxygen. These processes appeared to be mediated predominately by 1O2. This was further supported by the extremely high quantum yield of 1O2 generation by hypocrellin[21-23]s. Many investigations demonstrated that hypocrellins had a strong photodynamic effect on tumours[24] and impressive antiviral activity against human immunodeficiency virus type 1 (HIV-1)[25]. Recently, it has been reported that hypocrellin can photosensitize apoptotic cell death[26]. The above investigations collectively provide a compelling rationale for the development of hypocrellin and its derivatives as PDT photosensitizers.
Our group has recently isolated a filamentous fungal strain from western region of Yunnan Province in China and identified it as Ascomycetes Hypocreales Hypocreaceae Hypomyes(Fr) Tul.sp based on the taxonomic study. Hypomyces (Fr) Tul.Sp. was found for the first time to produce Elsinochrome A (EA), Hypocrellin A (HA) and Hypocrellin B (HB), under solid-phase fermentation conditions. Colorectal cancer is common in China. Since EA and Hypocrellins could be a potential tumor photopreventive and phototherapeutic agents, it is worthwhile to investigate the photodynamic effects of these photosensitizers. In this study, we examined the relative potency of EA, HA and HB against two cell lines, human colorectal carcinoma Hce-8693 cells and rhesus monkey embryonic stem ( ES ) R366.4 cells, and attempted to correlate anticancer activity with chemical structure and quantum yield of 1O2.
The fungal metabolites were isolated from solid-substrate fermentation cultures of Hypomyces (Fr) Tul.Sp. and evaporated to dryness. The powder of Hypomyces (Fr) Tul.Sp. was extracted with acetone at room temperature and then evaporated to dryness in vacuo. The recrystallized crude product was purified by silica gel column chromatography with a mixed solvent of petroleum ether:EtOAc:EtOH (4:2:1). The purified crystallized products were characterized with element analysis measurement (PE 2400), UV-visible spectrophotometry (PE UV/V is Lambda Bio), fluorescence spectra instruments (Hitachi-850), FT-IR(PE 1000), 1H, 13C-nuclear magnetic resonance (Bruker AM-400). The results were consistent with literature data.
Each of the above products was dissolved respectively in dimethylsulfoxide (DMSO) at 1 M and stored at 4°C in dark conditions. Under these conditions the solutions were stable for 2 months. The stock solutions were diluted 103 to 107 fold and in the final experimental conditions, the final DMSO concentration (0.1%) did not affect the viability of the culture cells, as demonstrated in control experiments.
Rhesus monkey embryonic stem cell line R366.4 was kindly provided by Dr James A Thomson (The Wisconsin Regional Primate Research Center, University of Wisconsin, US). Cells were plated in mouse embryonic fibroblasts (previously exposed to 3000 rads γ-radiation) in medium consisting of 85% Dulbecco’s Modified Eagle medium (4500 mg of glucose per liter, with L-glutamine, without sodium pyruvate; GIBCO) with 15% fetal bovine serum (HyClone), 1 × 10-7 Mol/L 2-mercaptoethanol (Sigma) and 1% nonessential amino acid stock (GIBCO). Human colorectal carcinoma Hce-8693 cells were obtained from ATCC. The cell lines Hce-8693 were maintained in Dulbecco’s Modified Eagle medium (GIBCO) supplemented with 10% new born calf serum (HyClone). All cell lines were grown at 37 °C under a water-saturated sterile atmosphere containing 5% CO2 (Forma Scientific Incubator). All cell manipulations in the presence of EA, HA and HB were performed under subdued light conditions.
Cells incubated with EA, HA and HB were irradiated with a water-cooled 1300 W tungsten-bromine lamp. All cells proliferated as monolayers attached to the plastic bottom of the plate which was completely transparent for the excitation light. Temperature recorded in tissue culture plate did not exceed room temperature during the irradiation period. Immediately after irradiation, cells were rinsed three times with PBS and grown in a fresh medium for 2 hours.
Cells were incubated with various doses of EA, HA or HB, irradiated, incubated for additional 2 h and then harvested, washed with phosphate-buffered saline (PBS) three times and fixed with 700 mL·L-1 ethanol at 4 °C overnight. Fixed cells were washed three times with PBS and stained with 800 μL propidium iodide and 200 μL deoxyribonulcease-free ribonuclease A in PBS. The fluorescence intensity of propidium iodide-stained nuclei was detected with flow cytometer (EPICS-XL, Coulter, USA) and 10000 cells were analyzed with Multicycle software.
R366.4 cells growing in sub-confluent culture were used to assess photocytotoxic effects of EA via flow cytometric assays. Graded doses of EA (1 × 10-7 Mol/L, 1 × 10-6 Mol/L, 1 × 10 -5 Mol/ L, 1 × 10-4 Mol/L, 1 × 10-3 Mol/L) dissolved in DMSO were mixed into the medium overlying 5.0×104 cells in 6-well plates. Following 2 h incubation, the cells were irradiated for 5 min, 6 min, 10 min and 20 min respectively (or not in case of darkness). After the drug-containing medium was removed, the cells were washed with phosphate-buffered saline (PBS) three times and the fresh ES culture medium was put on the cells prior to incubation for 2 h at 37 °C in saturated humidified air with 5% CO2. Finally, the cell proliferation was determined by flow cytometric assay.
Hce-8693 cells growing in confluent culture were used to assess inhibitory effects of EA, HA and HB via flow cytometric assays. For each compound, graded doses (1 × 10-6 Mol/L, 1 × 10-5 Mol/L, 1 × 10-4 Mol/L, 1 × 10-3 Mol/L) dissolved in DMSO were mixed into the medium overlying 5.0 × 104 cells in 6-well plates. Following 2 h incubation, the cells were irradiated for 5 min, 6 min, 10 min and 20 min respectively (or not in case of darkness). After the drug-containing medium was removed, the cells were washed with phosphate-buffered saline (PBS) three times and the fresh culture medium was put on the cells prior to an incubation for 2 h at 37 °C in saturated humidified air with 5% CO2. Finally, the cell proliferation was determined by flow cytometric assay.
Student’s t test was used to assess statistical significance of differences. If P < 0.01, the difference was considered very significant.
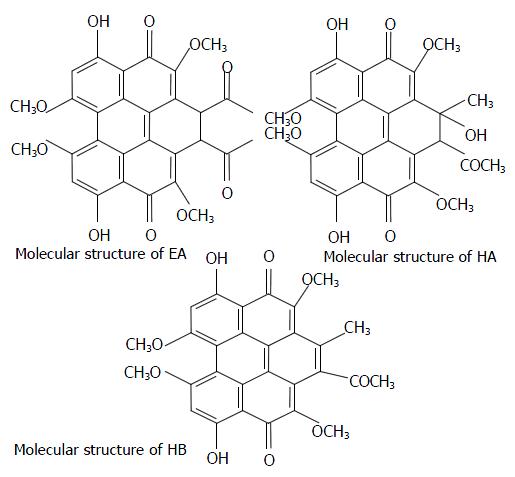
The structures of the compounds are shown in Figure 1, and their relevant photochemical properties are summarized in Table 1.
| Structure | UV λmax (log ε)* | λmax (log ε)* | φ 1O2 |
| EA | 459(1.60), 528(0.84), 568(1.04) | 460(3.78), 531(3.13), 571(3.60) | 0.94 |
| HA | 468(1.88), 542(0.83), 582(0.90) | 417(5.51), 542(1.02), 582(7.70) | 0.83 |
| HB | 470(0.27), 540(0.12), 583(0.13) | 471(4.39), 543(3.01), 583(3.36) | 0.76 |
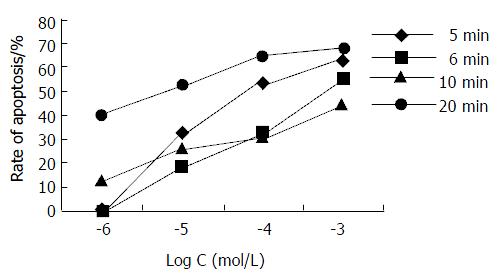
Embryonic stem (ES) cells are derived from preimplantation embryos, have a normal karyotype, and are capable of indefinite, undifferentiated proliferation[27]. Recently, in vitro mouse ES cell culture method has been used to test mutagenic, cytotoxic and embryotoxic effects of chemical substances[28-30]. In this study, rhesus monkey ES R366.4 cells were first used to measure the photocytoxicity of EA by judging the apoptosis of ES cells. After treated the R366.4 ES cells with EA at various concentrations, with or without light irradiation, the rate of apoptosis obtained by FCM were shown in Table 2 and Figure 2. The data illustrated that photoactivated EA had no cytotoxic effects on the R366.4 ES cells at low concentrations, which were 10-7 Mol/L, 10-6 Mol/L, 10-5 Mol/L respectively. Whereas, all of photoactivated EA at higher concentrations (10-4 Mol/L and 10-3 Mol/L respectively) exhibited a potent cytotoxic effects on R366.4 cells. In general, no large differences in the photodependent cytotoxic effects of EA were found between the different irradiation time. In the case of the photocytotoxic EA no cytotoxic effect was observed in dark conditions.
In order to investigate the antiproliferative effect of EA, Hce-8693 cells were incubated with different concentrations of EA under dark conditions and subjected 2 hours to different irradiation time (5, 6, 10 and 20 min respectively). The cells were then further incubated for an additional 2 hours in the dark without photosensitizer and measured via FCM assay. The rates of apoptosis induced by EA are shown in Table 3 and Figure 3. For each irradiation time, the data showed that there was dose-dependent relationship between EA doses and rate of Hce-8693 cell apoptosis. On the contrary, no large differences in the antiproliferative effect of the photoactivated EA was found between the different irradiation time.
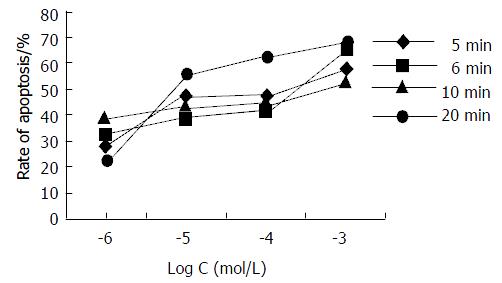
In order to investigate the antiproliferative effect of HA, Hce-8693 cells were incubated with different concentrations of HA under dark conditions and subjected 2 hours to different irradiation time (5, 6, 10 and 20 min respectively). The cells were then further incubated for additional 2 hours in the dark without photosensitizer and measured via FCM assay. The rates of apoptosis induced by HA were shown in Table 4 and Figure 4. For each irradiation time, the data showed that there was dose-dependent relationship between HA doses and rates of Hce-8693 cell apoptosis. On the contrary, no large differences in the antiproliferative effect of the photoactivated HA was found between the different irradiation time.
In order to investigate the antiproliferative effect of HB, Hce-8693 cells were incubated with different concentrations of HB under dark conditions and subjected 2 hours to different irradiation time (5, 6, 10 and 20 min respectively). The cells were then further incubated for additional 2 hours in the dark without photosensitizer and measured via FCM assay. The rates of apoptosis induced by HB were shown in Table 5 and Figure 5. For each irradiation time, the data showed that there was dose-dependent relationship between HB doses and rate of Hce-8693 cell apoptosis. On the contrary, no large differences in the antiproliferative effect of the photoactivated HB was found between the different irradiation time.
From the results of photodependent cytotoxicity studies in R366.4 cell lines, it seemed that photoactivated EA had no cytotoxic effects at 10-6 Mol/L and 10-5 Mol/L concentrations. On the contrary, EA, HA and HB exhibited more of less antiproliferative effects on human Hce-8693 cells at this range of concentrations. Thus, the photodynamic effects of the photosensitizers could be compared from the rate of apoptosis (Table 6). The order of efficiency would be approximately HA > EA > HB (Figure 6).
| Group | Rate of apoptosis/% | |||||
| 10-6 Mol/L | 10-5 Mol/L | |||||
| HA | EA | HB | HA | EA | HB | |
| 5 min | 0 | 0 | 0 | 47.2 ± 8.79 | 32.7 ± 7.56 | 0 |
| 6 min | 32.0 ± 5.64 | 0 | 0 | 39.1 ± 6.41 | 19.3 ± 4.16 | 0 |
| 10 min | 40.2 ± 6.23 | 13.4 ± 3.25 | 0 | 45.2 ± 8.40 | 26.4 ± 4.89 | 20.0 ± 4.21 |
| 20 min | 22.6 ± 3.39 | 40.5 ± 8.58 | 13.7 ± 3.02 | 56.6 ± 3.86 | 52.7 ± 11.82 | 13.9 ± 2.87 |
Photodynamic therapy (PDT) is a medical treatment based on the use of a sensitizer to promote photoinduced damage to biological molecules including lipids, proteins and DNA[31,32]. It can be used to eradicate early localized tumours and for palliation of more advanced disease when metastasis has occurred. This treatment modality involves the use of light in combination with a photosensitizing compound. Following excitation of photosensitizers to long-lived excited singlet and/ or triplet states, the tumour is destroyed either by reactive oxygen species (Type II mechanism) and/or by radical products (Type I mechanism)[33-37].
Hypocrellins are efficient singlet oxygen generators during photochemical reactions and may also exert photosensitization via radical mechanisms, which may confer a degree of independence from classical oxygendependent photochemical mechanisms. This feature is important in the context of impaired radiosensitivity and chemosensitivity of hypoxic human tumour cells. However, the precise mode of action of these molecules at the cellular level is not clear and seems to go far beyond Type I and Type II photoprocesses[38-42]. An additional mechanism involving protons released in the excited state and leading to cellular pH drop has also been proposed for the related pigments hypocrellin and hypericin[43].
Apoptosis is a complex and programmed process which is regulated by a variety of factors. Recently, it has been reported that hypocrellins and their derivatives can photosensitize apoptotic cell death[44,45]. However, the molecular mechanisms of tumor cell apoptosis induction by Hypocrellin A and B are poorly understood. The antiproliferative actions of hypocrellin may be due, in part, to the ability of hypocrellin A to induce reactive oxygen species (Type II mechanism)[46,47]. In addition, hypocrellin A-mediated apoptosis is increased when antisense bcl-2 retrovirus vector is transfected into human gastric adenocarcinoma MGC803 cells[48]. And also, Ali et al reported that caspase-3 and hydrogen peroxide may play an important role in HA and HB-induced apoptosis[49,50].
According to the photochemical properties, the quantum yields of EA, HA and HB are 0.94, 0.83, 0.76 respectively. From the results of inhibitory effect of EA, HA and HB on the proliferation of Hce-8693 cells, it seems that the order of efficiency would be approximately HA > EA > HB. In this way, the molecular mechanisms of Hce-8693 cell apoptosis induction by EA, HA and HB may not be induced by reactive oxygen species (Type II mechanism). It is also noteworthy that photoactivated EA, HA and HB can selectively inhibit the growth of human colorectal carcinoma cells but not rhesus monkey embryonic stem R366.4 cells at lower concentrations. Thus, the molecular mechanisms of apoptosis induced by photoactivated EA, HA and HB are worth further investigation.
Edited by Xu JY
| 1. | Chen CT, Nakanishi K, Natori S. Biosynthesis of elsinochrome A, the perylenequinone from Elsinoë spp. I. Chem Pharm Bull (Tokyo). 1966;14:1434-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Weisgraber KH, Weiss U. Pigments of Elsinoe species. VI. A simple synthesis of a related perylenequinone. J Chem Soc Perkin 1. 1972;1:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Stack ME, Mazzola EP, Page SW, Pohland AE, Highet RJ, Tempesta MS, Corley DG. Mutagenic perylenequinone metabolites of Alternaria alternata: altertoxins I, II, and III. J Nat Prod. 1986;49:866-871. [PubMed] [DOI] [Full Text] |
| 4. | Davis VM, Stack ME. Mutagenicity of stemphyltoxin III, a metabolite of Alternaria alternata. Appl Environ Microbiol. 1991;57:180-182. [PubMed] |
| 5. | Xu S, Chen S, Zhang M, Shen T. A novel method for the preparation of amino-substituted hypocrellin B. Bioorg Med Chem Lett. 2001;11:2045-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Ma JS, Yan F, Wang CQ, An JY. Hypocrellin-A sensitized photooxidation of bilirubin. Photochem Photobiol. 1989;50:827-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Miller GG, Brown K, Ballangrud AM, Barajas O, Xiao Z, Tulip J, Lown JW, Leithoff JM, Allalunis-Turner MJ, Mehta RD. Preclinical assessment of hypocrellin B and hypocrellin B derivatives as sensitizers for photodynamic therapy of cancer: progress update. Photochem Photobiol. 1997;65:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | He YY, An JY, Jiang LJ. Glycoconjugated hypocrellin: synthesis of [(beta-D-glucosyl)ethylthiyl]hypocrellins and photosensitized generation of singlet oxygen. Biochim Biophys Acta. 1999;1472:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Daub ME, Ehrenshaft M. THE PHOTOACTIVATED CERCOSPORA TOXIN CERCOSPORIN: Contributions to Plant Disease and Fundamental Biology. Annu Rev Phytopathol. 2000;38:461-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Yu C, Chen S, Zhang M, Shen T. Spectroscopic studies and photodynamic actions of hypocrellin B in liposomes. Photochem Photobiol. 2001;73:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Ververidis P, Davrazou F, Diallinas G, Georgakopoulos D, Kanellis AK, Panopoulos N. A novel putative reductase (Cpd1p) and the multidrug exporter Snq2p are involved in resistance to cercosporin and other singlet oxygen-generating photosensitizers in Saccharomyces cerevisiae. Curr Genet. 2001;39:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Wang SS, Mathes C, Thompson SH. Membrane toxicity of the protein kinase C inhibitor calphostin A by a free-radical mechanism. Neurosci Lett. 1993;157:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Gamou S, Shimizu N. Calphostin-C stimulates epidermal growth factor receptor phosphorylation and internalization via light-dependent mechanism. J Cell Physiol. 1994;158:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Pedron T, Girard R, Inoue K, Charon D, Chaby R. Lipopolysaccharide and the glycoside ring of staurosporine induce CD14 expression on bone marrow granulocytes by different mechanisms. Mol Pharmacol. 1997;52:692-700. [PubMed] |
| 15. | Dubauskas Z, Beck TP, Chmura SJ, Kovar DA, Kadkhodaian MM, Shrivastav M, Chung T, Stadler WM, Rinker-Schaeffer CW. Activated calphostin C cytotoxicity is independent of p53 status and in vivo metastatic potential. Clin Cancer Res. 1998;4:2391-2398. [PubMed] |
| 16. | Chen CL, Chen H, Zhu DM, Uckun FM. Quantitative high-performance liquid chromatography-based detection method for calphostin C, a naturally occurring perylenequinone with potent antileukemic activity. J Chromatogr B Biomed Sci Appl. 1999;724:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Chen CL, Tai HL, Zhu DM, Uckun FM. Pharmacokinetic features and metabolism of calphostin C, a naturally occurring perylenequinone with antileukemic activity. Pharm Res. 1999;16:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Meille SV, Malpezzi L, Allegra G, Nasini G, Weiss U. Structure of elsinochrome A: a perylenequinone metabolite. Acta Crystallogr C. 1989;45:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Ma JS, Yan F, Wang CQ, An JY. Hypocrellin-A sensitized photooxidation of bilirubin. Photochem Photobiol. 1989;50:827-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Diwu Z, Zimmermann J, Meyer T, Lown JW. Design, synthesis and investigation of mechanisms of action of novel protein kinase C inhibitors: perylenequinonoid pigments. Biochem Pharmacol. 1994;47:373-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Fehr MJ, Carpenter SL, Wannemuehler Y, Petrich JW. Roles of oxygen and photoinduced acidification in the light-dependent antiviral activity of hypocrellin A. Biochemistry. 1995;34:15845-15848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Hirayama J, Ikebuchi K, Abe H, Kwon KW, Ohnishi Y, Horiuchi M, Shinagawa M, Ikuta K, Kamo N, Sekiguchi S. Photoinactivation of virus infectivity by hypocrellin A. Photochem Photobiol. 1997;66:697-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Park J, English DS, Wannemuehler Y, Carpenter S, Petrich JW. The role of oxygen in the antiviral activity of hypericin and hypocrellin. Photochem Photobiol. 1998;68:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Diwu Z. Novel therapeutic and diagnostic applications of hypocrellins and hypericins. Photochem Photobiol. 1995;61:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 252] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Hudson JB, Zhou J, Chen J, Harris L, Yip L, Towers GH. Hypocrellin, from Hypocrella bambuase, is phototoxic to human immunodeficiency virus. Photochem Photobiol. 1994;60:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 135] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Zhang J, Cao EH, Li JF, Zhang TC, Ma WJ. Photodynamic effects of hypocrellin A on three human malignant cell lines by inducing apoptotic cell death. J Photochem Photobiol B. 1998;43:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA. 1995;92:7844-7848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 664] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 28. | Schleger C, Krebsfaenger N, Kalkuhl A, Bader R, Singer T. [Innovative cell culture methods in drug development]. ALTEX. 2001;18:5-8. [PubMed] |
| 29. | Rohwedel J, Guan K, Hegert C, Wobus AM. Embryonic stem cells as an in vitro model for mutagenicity, cytotoxicity and embryotoxicity studies: present state and future prospects. Toxicol In Vitro. 2001;15:741-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Genschow E, Spielmann H, Scholz G, Seiler A, Brown N, Piersma A, Brady M, Clemann N, Huuskonen H, Paillard F. The ECVAM international validation study on in vitro embryotoxicity tests: results of the definitive phase and evaluation of prediction models. European Centre for the Validation of Alternative Methods. Altern Lab Anim. 2002;30:151-176. [PubMed] |
| 31. | Xu Y, Zhao H, Zhang Z. Raman spectroscopic study of microcosmic and photosensitive damage on the liposomes of the mixed phospholipids sensitized by hypocrellin and its derivatives. J Photochem Photobiol B. 1998;43:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | He YY, Jiang LJ. Photosensitized damage to calf thymus DNA by a hypocrellin derivative: mechanisms under aerobic and anaerobic conditions. Biochim Biophys Acta. 2000;1523:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Fu NW. [Advances in research on photosensitizers]. Shengli Kexue Jinzhan. 1992;23:36-40. [PubMed] |
| 34. | Estey EP, Brown K, Diwu Z, Liu J, Lown JW, Miller GG, Moore RB, Tulip J, McPhee MS. Hypocrellins as photosensitizers for photodynamic therapy: a screening evaluation and pharmacokinetic study. Cancer Chemother Pharmacol. 1996;37:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Diwu ZJ, Haugland RP, Liu J, Lown JW, Miller GG, Moore RB, Brown K, Tulip J, McPhee MS. Photosensitization by anticancer agents 21: new perylene- and aminonaphthoquinones. Free Radic Biol Med. 1996;20:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Wang ZJ, He YY, Huang CG, Huang JS, Huang YC, An JY, Gu Y, Jiang LJ. Pharmacokinetics, tissue distribution and photodynamic therapy efficacy of liposomal-delivered hypocrellin A, a potential photosensitizer for tumor therapy. Photochem Photobiol. 1999;70:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Wu T, Xu S, Shen J, Song A, Chen S, Zhang M, Shen T. New potential photodynamic therapeutic anti-cancer agents: synthesis and characterization of demethoxy amino-substituted hypocrellins. Anticancer Drug Des. 2000;15:287-293. [PubMed] |
| 38. | Nenghui W, Zhiyi Z. Relationship between photosensitizing activities and chemical structure of hypocrellin A and B. J Photochem Photobiol B. 1992;14:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Yuying H, Jingyi A, Lijin J. Effect of structural modifications on photosensitizing activities of hypocrellin dyes: EPR and spectrophotometric studies. Free Radic Biol Med. 1999;26:1146-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Datta A, Smirnov AV, Wen J, Chumanov G, Petrich JW. Multidimensional reaction coordinate for the excited-state H-atom transfer in perylene quinones: importance of the 7-membered ring in hypocrellins A and B. Photochem Photobiol. 2000;71:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Wu T, Shen J, Song A, Chen S, Zhang M, Shen T. Photodynamic action of amino substituted hypocrellins: EPR studies on the photogenerations of active oxygen and free radical species. J Photochem Photobiol B. 2000;57:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Xu S, Shen J, Chen S, Zhang M, Shen T. Active oxygen species (1O2, O2*-) generation in the system of TiO2 colloid sensitized by hypocrellin B. J Photochem Photobiol B. 2002;67:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Chaloupka R, Sureau F, Kocisova E, Petrich JW. Hypocrellin A photosensitization involves an intracellular pH decrease in 3T3 cells. Photochem Photobiol. 1998;68:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Ali SM, Chee SK, Yuen GY, Olivo M. Hypericin and hypocrellin induced apoptosis in human mucosal carcinoma cells. J Photochem Photobiol B. 2001;65:59-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Ali SM, Olivo M, Yuen GY, Chee SK. Photodynamic-induced apoptosis of human nasopharyngeal carcinoma cells using Hypocrellins. Int J Oncol. 2001;19:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Ma J, Jiang L. Photogeneration of singlet oxygen (1O2) and free radicals (Sen*-, O2*-) by tetra-brominated hypocrellin B derivative. Free Radic Res. 2001;35:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Wu T, Xu S, Shen J, Chen S, Zhang M, Shen T. EPR investigation of the free radicals generated during the photosensitization of TiO2 colloid by hypocrellin B. Free Radic Res. 2001;35:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Zhang WG, Ma LP, Wang SW, Zhang ZY, Cao GD. Antisense bcl-2 retrovirus vector increases the sensitivity of a human gastric adenocarcinoma cell line to photodynamic therapy. Photochem Photobiol. 1999;69:582-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Ali SM, Chee SK, Yuen GY, Olivo M. Hypocrellins and Hypericin induced apoptosis in human tumor cells: a possible role of hydrogen peroxide. Int J Mol Med. 2002;9:461-472. [PubMed] |
| 50. | Ali SM, Chee SK, Yuen GY, Olivo M. Photodynamic therapy induced Fas-mediated apoptosis in human carcinoma cells. Int J Mol Med. 2002;9:257-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |