INTRODUCTION
A major hurdle in most current gene therapy lies in how to transfer genes to target tissues efficiently and how to obtain therapeutic expression of target genes. The targeting efficacy of gene transfection and expression directly determines whether the gene therapy has high efficiency and whether it is harmful to normal tissues. Therefore, how to enhance the targeting efficacy of gene delivery has become a problem to be solved urgently[1-4].
At present, various gene delivery methods have been used in gene therapy[5-8]. Among them, there are two important methods to achieve tumor-targeting gene therapy: one is receptor-mediated gene delivery; another is the construction of tumor-targeting gene expression vector which utilizes tumor-specific transcription regulatory sequence[9-12]. However, either of them, if used singly, can not really accomplish the tumor-targeting gene therapy.
This paper reported a novel HBV antisense RNA delivery system targeting on hepatocarcinoma cells, named AFP enhancing 4-element complex. This complex includes four elements: (1) recombinant EB virus vector, pEBAF-as-preS2, in which antisense preS2 gene was cloned under the control of human α-fetoprotein (AFP) promoter and enhancer, enabled preS2 antisense RNA to be expressed specifically in AFP-positive cells; (2) ligand oligopeptide GE7, synthesized according to the putative binding region of epidermal growth factor (EGF) to its receptor (EGFR), could transfer DNA specifically to EGFR positive cells; (3) HA20, a homologue of N-terminus of haemagglutin of Influenza envelope protein, was synthesized as endosome-releasing oligopeptide (EROP); (4) polylysin was polycation peptides (PCP) which could interact with pEBAF-as-preS2 DNA to form a complex after conjugation with GE7 and HA20. Both in vitro and in vivo studies indicated that preS2 antisense RNA could be expressed strictly and specifically in hepatocarcinoma cells after being transfected by this complex. This study implicated the great therapeutic potential of AFP-enhancing 4-element complex for HBV-associated hepatocelluar carcinoma.
MATERIALS AND METHODS
Cells and cultures
Hepatocelluar carcinoma cell line HepG2.2.15 integrated with whole HBV gene not only can accomplish the transcription and translation of HBV, but also may produce Dane’s particles. This cell line, provided by BeiJing Institute of Medicine and Biology, was cultured in the MEM medium containing 10% FCS and 380 μg/mL G418.The human hepatocelluar carcinoma cell line BEL7402 was cultured in the DMEM medium with 10% FCS. The human umbilical vein endothelial cell line ECV304 contributed by Chinese Academy of Military Medical science was cultured in the DMEM medium, containing 10% FCS and diantibiotics. The medium mentioned above were purchased from Hyclone Co. American, serum from GIBCO Co.
Animals
BALB/C nude mice, male, 5-6 weeks of age, were purchased from experimental animal center of TongJi medical college, Central China Science and Technology University, WuHan. 1 × 107 HepG2.2.15 cells were transplanted subcutaneously into nude mice and were ready for use when the tumor size reached about 0.5 cm in diameter.
Plasmid
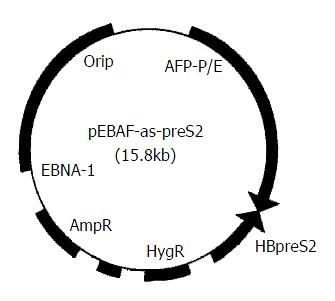
HBV preS2 gene (3203-3340) was cloned reversely downward 5.5 kb of AFP promoter and enhancer to construct hepatocarcinoma specific preS2 antisense RNA expression vector, pEBAF-as-preS2 (Figure 1) which could express antisense RNA against preS2 in AFP-positive cells. (published elsewhere).
Figure 1 Physical map of pEBAF-as-preS2.
hepatocarcinoma specific preS2 antisense RNA expression vector, pEBAF-as-preS2 was constructed by inserting HBV preS2 gene (3203-3340) reversely downward the AFP promoter and enhancer (AFP-P/E) of plasmid pEBAF which contains the key element of EB virus duplication (Ori P and EBNA-1).
Synthesis of GE7-PL and HA20-PL[
13]
GE7, a 16-peptide specific to EGFR, and HA20,a homologue of N-terminus of haemagglutinin of influenza viral envelope protein, were synthesized by National Laboratory for Oncogene and Related Genes, Shanghai Cancer Institute. GE7 and HA20 were conjugated with polylysine (PL) respectively (GE7-PL and HA20-PL).
Preparation of AFP-enhancing 4-element complex
pEBAF-as-preS2 DNA, GE7-PL and HA20-PL conjugates were respectivety mixed dropwise into 10 μL of deionizeded water in different ratios. The mixture was incubated at 25 °C for 30 min. A mixture containing 0.2 μg plasmid DNA was analyzed on 1% agrose gel eletrophoresis to examine the retardation of DNA migration, in order to determine the optimal ratio. According to the optimal ratio of DNA to conjugates based on above experiment, the plasmid DNA was mixed dropwise to the conjugates of GE7-PL and HA20-PL and reacted at 25 °C for 30 min. Examine the retardation of DNA by 1% agrose gel eletrophoresis. Then the complex could be used for gene transfection.
In vitro gene transfer by 4-element complex
5 × 104 cells were seeded into 0.5 mL medium in each well of a 24 well plate. After cell density reached a confluence of 60%, the medium was removed and replaced with 0.5 mL of complete medium containing 4-element complex in a quantity equivalent to 0.2 μg DNA. Cells were cultured at 37 °C and incubated with 5% CO2 overnight, then replaced with fresh complete MEM medium without 4-element complex and cultured at 37 °C with 5% CO2. 3 days after, the supernatant was collected to detect the HBV antigen. Non-transfected HepG2.2.15 cells were used as negative control.
Detection of virus antigens
According to the protocol of antigen detection kit (Lizhu Co. ShenZhen, China), the concentration of HBsAg and HBeAg in the supernatant were detected by ELISA. The results were illustrated with P/N value (P/N = sample A/negative control A; A stands for the amount of light absorbent).
In vivo gene transfection by AFP-enhancing 4-element complex
AFP-enhancing 4-element complex containing 0.2 μg DNA were injected into a tail vein of nude mice bearing 0.5 cm tumor. Animals were sacrificed 3 days after injection, then the tumor, liver, spleen, kidney, stomach were dissected and used for extracting the total RNA.
Extraction of total RNA
Total RNA was extracted from 5 mm3 fresh mice tissues according to the protocol of TRIZOL kit. Products were quantitated by Biophotometer (Eppendorf Co.).
Primer design
According to the sequence described by Ono et al in 1986[14], the primers were designed to amplify HBpreS2 gene: forward (S2P1): 5’GCTCTAGACTCAGGCCATGCATG3’; reverse (S2P2): 5’GCTCTAGATGGTGAGTGATTGGAGGT3’. The primers for β-actin, a housekeeping gene, were designed simultaneously (forward: 5’ACACTGTGCCCA 3’; reverse: 5’ATGATGGAGTTGAAGGTAGTTTCGTGGAT3’). All of these oligonucleotide primers were synthesized by Shanghai Biology and Engineering Company.
RT-PCR
Reverse transcription was performed in 20 μL volume including: 1 μg of total RNA, 4 μL of 5 × buffer, 20 nmol dNTP, 20 Units of RNase inhibitor and 200 Units of Moloney murine leukemia virus (M-MLV) reverse transcriptase. The reverse transcription reaction was performed at 42 °C for 1hr.The products were denatured at 70 °C for 10 min and then preserved at 4 °C. cDNA was used as templates for the amplification of preS2 with primers S2P1 and S2P2. PCR was carried out in 25 μL volume with 1 μL of cDNA, 2.5 μL of 10 × buffer, 0.3 mmol Mg++, 5nmol/L dNTP, 3 Units of Taq polymerase, 10 nmol/L primers (forward and reverse). Reaction conditions were: 94 °C for 5 min, then 35 cycles at 94 °C for 40 sec, 50 °C for 40 sec, 72 °C for 50 sec, followed by a final extension period of 7 min at 72 °C.
In vivo tumor-inhibitory experiments
100 μL AFP-enhancing 4-element complex containing 0.2 μg pEBAF-as-preS2 DNA were injected into tumors of nude mice bearing hepatocarcinoma cells HepG2.2.15, once each week for 4 weeks and physiological salt solution was injected likewise in the control group. The diameters of tumors were measured every 3 days. Animals were sacrificed 1 week after the final injection. Tumor tissues were dissected and their diameters were measured.
RESULTS
Construction of AFP-enhancing 4-element complex
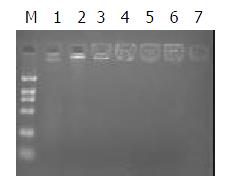
Polypeptide and plasmid were mixed in different ratios. According to the results of 1% agrose gel eletrophoresis, DNA was completely trapped at the slot with no DNA migration when the ratio was 1:1 (Figure 2). This is the optimal ratio of polypeptide to pEBAF-as-preS2 plasmid DNA.
Figure 2 Construction of AFP-enhancing 4-element complex.
0.2 μg plasmid DNA was independently mixed dropwise with various quantity of polypeptide conjugation for 30 min at room temperature, and then the mixture was analyzed with 1% agrose gel electrophorosis. Fig 2 showed the DNA retardation of different complex mixed with DNA and peptide in differ-ent ratios. The ratio of DNA and peptide in Lane 1 to 7 is 0, 1/ 1,1/2. 1/2.5, 1/3, 1/3.5. 1/4. M indicates DNA marker.
Extraction of total RNA
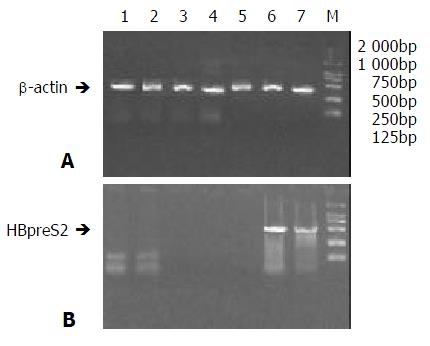
Total RNA from all biopsies were analysed by Biophotometer (Eppendorf Co.). The ratios of A260 to A280 were all between 1.8 to 2.0. Using above total RNA as templates, RT-PCR were performed with primers for β-actin gene. A band of about 300bp in size was obtained in agrose gel eletrophoresis (Figure 3A), proving that RNA was qualified for RT-PCR.
Figure 3 RT-PCR of tissues in nude mice treated AFP-enhanc-ing 4-element complex.
Total RNAs of different tissues of Nude mice bearing hepatocarcinoma were extracted 3 days after treatmemt with AFP-enhancing 4-element complex and then RT-PCR was performed; the expression of HBV preS2 gene was analyzed. A. RT-PCR of beta-actin. (bands 1 to 7 stand for the PCR products of spermaduct, stomach, liver, spleen, heart, and tumors, M stands for the DNA marker); B. RT-PCR of HBV preS2 gene.
Hepatocarcinoma directed expression of antisense RNA mediated by AFP-enhancing 4-element complex
RT-PCR was carried out with specific primers (S2P1, S2P2) for HBpreS2 gene. 2% gel electrophoresis demonstrated that the products of amplification could be detected only in tumor tissues of animals injected with AFP-enhancing 4-element complex. No specific bands could be tested in other tissues, especially in the epithelial cells of stomach mucosa and spermaduct. (Figure 3B).
Inhibitory effects of AFP-enhancing 4-element complex on expression of HBV antigen in hepatocarcinoma cells
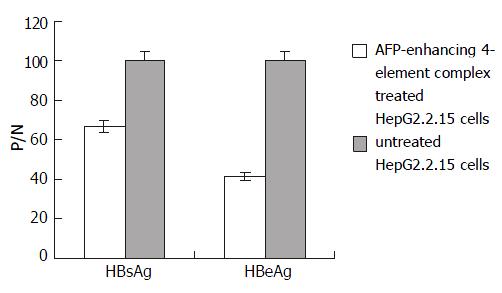
In order to correct the results, the supernatants of cell cultures in all groups were collected before transfection, marked as P/N-0hr. The final results were calculated according to the following formula: antigen secretion of cells transfected with AFP-enhancing 4-element (tested) = [P/N of tested]/([P/N-0hr of tested] × [P/N-0hr of control]). HBsAg and HBeAg expressions of HepG2.2.15 were lowered prominently after transfection with AFP-enhancing 4-element complex. The inhibitory rates were 33.4% and 58.5% respectively (Figure 4).
Figure 4 Effect of AFP-enhancing 4-element complex on HBV gene expression in HepG2.
2.15 cells.
In vivo inhibitory effects of AFP-enhancing 4-element complex on growth of hepatocellular carcinoma in nude mice
After 4 injections of AFP-enhancing 4-element complex containing 0.2 μg DNA, the diameter of tumor tissue was 0.995 cm ± 0.35, which was significantly smaller than that of the control groups (2.215 cm ± 0.25, P < 0.05).
DISCUSSION
Hepatocellular carcinoma (HCC), especially HBV-associated HCC, is one of the most common malignant tumor in China. At present, there’s still no efficient therapy methods[15-26]. Antisense nucleic acid, blocking the target gene and inhibiting its expression at the molecular level, has potential antiviral and antitumor effects[27-31]. In recent years, great interests have been focusing on such novel gene therapy. Compared with synthesized antisense DNA, antisense RNA could be expressed constantly in vivo, so that it has long-term antiviral effects. Thus, antisense RNA is an ideal choice in gene therapy for HBV[32-34].
There are three crucial factors to determine the efficacy of antisense gene therapy[35]: whether the target gene plays a crucial role in tumorgenesis, whether it could be transfected uniquely to target cells, and whether it could be expressed efficiently and specifically in those target cells. In this research, according to the characteristics of HBV-associated HCC, we selected preS2 which might play an important role in the pathogenetic targete site to be blocked. Moreover, in order to express preS2 antisense RNA strictly targeting on liver tumor cells, virus expression vector containing hepatocarcinoma-specific regulatory sequences (AFP promoter and enhancer) and EROP (HA20) combined with receptor mediated gene delivery system was prepared.
The protein encoded by preS2 gene plays an important role in the infection as well as malignant transformation of HBV[36,37]. In a previous research, we have proved that synthesized antisense oligodeoxynucleotids (asON) against translation initiation region (3203-3219) of HBpreS2 has the strongest inhibitory effect on the expression of HBV antigen in HepG2.2.15 cells in vitro[38]. In addition, AS5, designed by Putlitz[33] in this region, could efficiently inhibit the viral replication and the expression of HBV antigen. In this paper, preS2 gene (3203-3240nt) was selected as targete site for blocking by antisense RNA. Inhibitory experiments in vitro had demonstrated that a block of preS2 gene (3203-3240) could efficiently inhibited the expression of HBV antigen in HepG 2.2.15 cells. The inhibitory rates of the expression of HBsAg and HBeAg were 33.4% and 58.5% respectively.
The receptor-mediated gene delivery system transfers genes into the targete cells by endocytosis via specific receptor. In 1998, Tian, Gu et al (Shanghai Cancer Institute)[13] established a novel high-efficiency receptor-mediated gene delivery system in which GE7 targeted on EGFR were used to transfer genes to tumor cells, and HA20 was synthesized as an EROP to enable the exogeneous genes to escape from the lysosome. These properties guaranteed this system to have a significant potential in gene therapy.
However, the expression of EGFR is not limited to tumor cells. We have demonstrated that green fluorescence protein (GFP) reporter gene could be also expressed in the epithelial cells of stomach mucosa and spermaduct after being transfected with 4-element complex in vivo (published elsewhere).
For this reason, hepatocarcinoma tissue-specific antisense RNA expression vector under the control of AFP promoter was used to conjugate with GE7-PL and HA20-PL to construct an AFP-enhancing 4-element complex. As a specific marker for primary hepatocellular carcinoma, alfa fetal protein (AFP) possesses a high-targetable transcription-regulatory sequence which could be regulated precisely[39]. Therefore, it could direct the therapeutic gene to be specifically expressed in AFP-positive hepatocellular carcinoma cells. Using human AFP in-cis element (promoter and enhancer) to control the expression of antisense RNA could not only enhance the high efficacy, but also possesses the targetability to hepatocellular carcinoma cells. Results showed that preS2 antisense RNA was only examined in tumor tissues of nude mice injected with AFP-enhancing 4-element complex but could not be examined in the epithelial cells of stomach mucosa and spermaduct either. Morever, the growth of hepatocellular carcinoma in nude mice could be thwarted distinctively by being injected with AFP-enhancing 4-element complex at a dose equivalent of 0.2 μg DNA per mouse each time for 4 times.
In summary, this experiment showed that AFP-enhancing 4-element complex could deliver preS2 antisense RNA targeted on hepatoma cells and inhibited both HBV gene expression in vitro and tumor growth in vivo. All these results indicated the therapeutic potential of AFP-enhancing 4-element complex for HBV related HCC.