Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2198
Revised: April 7, 2003
Accepted: April 14, 2003
Published online: October 15, 2003
AIM: To evaluate the role of multiphasic scanning by multirow-detector helical CT (MDCT) in detecing small hypervascular hepatocellular carcinoma (SHCC).
METHODS: Multiphasic scanning was carried out in 75 patients with SHCC with Marconi MX8000 CT scanner. The early arterial phase (EAP), late arterial phase (LAP) and the portal venous phase (PVP) scans were started at 21 s, 34 s and 85 s respectively. The mean difference of CT values between tumor and liver parenchyma for each scanning phase was measured, and the sensitivity of detection of SHCC in each of these phases and in the combined phase was calculated and statistically analyzed.
RESULTS: The mean difference of CT values between tumor and liver parenchyma was significant in 71 lesions ≥ 1 cm in three phases (P < 0.05). In 91 tumor foci, the detectability of SHCC was 45.1%, 83.5% and 92.3% in EAP, LAP and double arterial phases (DAP), respectively. The early arterial phase plus the portal venous phase and the double arterial phase plus the portal venous phase were 94.5%, 97.8%, respectively. Whereas the detectability in LAP plus PVP and in DAP plus PVP had no statistical difference.
CONCLUSION: The utility of faster speed and thinner slice MDCT and multiphase scanning protocol can improve the detectability of hypervascular small hepatocellular carcinoma. Among which LAP is superior to EAP in depicting the lesions.
- Citation: Zhao H, Zhou KR, Yan FH. Role of multiphase scans by multirow-detector helical CT in detecting small hepatocellular carcinoma. World J Gastroenterol 2003; 9(10): 2198-2201
- URL: https://www.wjgnet.com/1007-9327/full/v9/i10/2198.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i10.2198
It has been recognized that the majority of hepatocellular carcinomas (HCC) are hypervascular. During the hepatic arterial phase (AP), hypervascular lesions will be greatly enhanced, and become iso- or hypodense in the portal venous phase (PVP), which is a sensitive and specific feature for diagnosing SHCC. A biphasic hepatic acquisition helical CT scanning technique has become a standard method for clinical diagnosis of SHCC. Some studies and clinical applications have shown that the arterial dominant phase using a single row detector helical CT (SDCT) is effective for detection of SHCC[1-6], but by using this kind of scanner, the scanning of the whole liver takes approximately 20 s with a great difference in scanning time from subdiaphragmatic region to the right lower border of the liver. So that, some lesions are not conspicuously enhanced within the time.
Recently, a new generation of MDCT has been used in clinical practice. The scanning time can be shortened to 0.5 s. If the four-detector array CT scanner is used, the entire hepatic acquisition can be accomplished in a very short period (4-8 s). Therefore, to makes use of the advantages of faster scanning speed of MDCT, we can create a double arterial phase (DAP) scanning technique and compare the detectability of SHCC in the early arterial phase (EAP), late arterial phase (LAP) and portal venous phase (PVP).
From September 2001 to July 2002, 75 patients (67 men, 8 women, mean age 49 years) with 91 lesions were enrolled in this study. All the patients were proved or suspected to have SHCC who had undergone imaging diagnostics as US, SDCT or MRI. Among them, 40 cases were confirmed by surgical operation or biopsy, 11 recurred cases were postoperatively diagnosed, and the other 24 cases were diagnosed by clinical data, such as history of liver diseases, evaluation of serum AFP and other imaging modalities.
Multiphasic CT scans of liver were performed with Marconi MX8000 CT scanner using the following parameters, namely 0.5-0.75 s scanning time, 6.5 mm thick section, 23.3 mm/s table speed, 120 KVp and 200-250 mA.
Before examination, 800-1000 mL water was taken as oral contrast. The whole liver scanning was followed by nonionic contrast enhancement with a dose of 1.5 mL/kg and an injection rate of 3 mL/s via the antecubital vein. Multiphase acquisition was performed with a scanning delay set for EAP, LAP and PVP at 21 s, 34 s and 85 s, respectively. Each of the whole liver scanning by cephalad-caudal orientation was completed in 4-8 s with breath held.
CT attenuations of 71/91 tumor foci with diameter ≥ 1 cm were measured in all images, and the difference in density of the tumor foci and surrounding hepatic parenchyma was calculated during the enhanced three phases.
Based on the enhancement and the comparison with adjacent liver parenchyma, the tumor foci were described as hyper-, iso- and hypoattenuation, only hyper- and hypoattenuation lesions could be detected and the number of tumor foci was recorded blindly by two radiologists.
The statistical analysis was done by SPSS10.0 software, Chi-square test and analysis of variance.
Among the ninety-one tumor foci identified on the images, 20 of them were less than 1 cm in diameter. The size of the other 71 lesions varied from 10 mm to 30 mm (mean 21.3 mm.) The maximum contrast of tumor-to-liver of the majority lesions during the late arterial phase is shown in Table 1. The difference of tumor-to-liver contrast among the EAP, LAP and PVP was statistically significant (P < 0.05).
The sensitivity of tumor foci detection in each scanning phase is shown in Table 2.
| Scanning phases | Sensitivity (%) (n = 91) |
| EAP | 45.1 (41/91) |
| LAP | 83.5 (76/91) |
| DAP | 92.3 (84/91) |
| PVP | 78.0 (71/91) |
| EAP + PVP | 84.6 (77/91) |
| LAP + PVP | 94.5 (86/91) |
| EAP + LAP + PVP | 97.8 (89/91) |
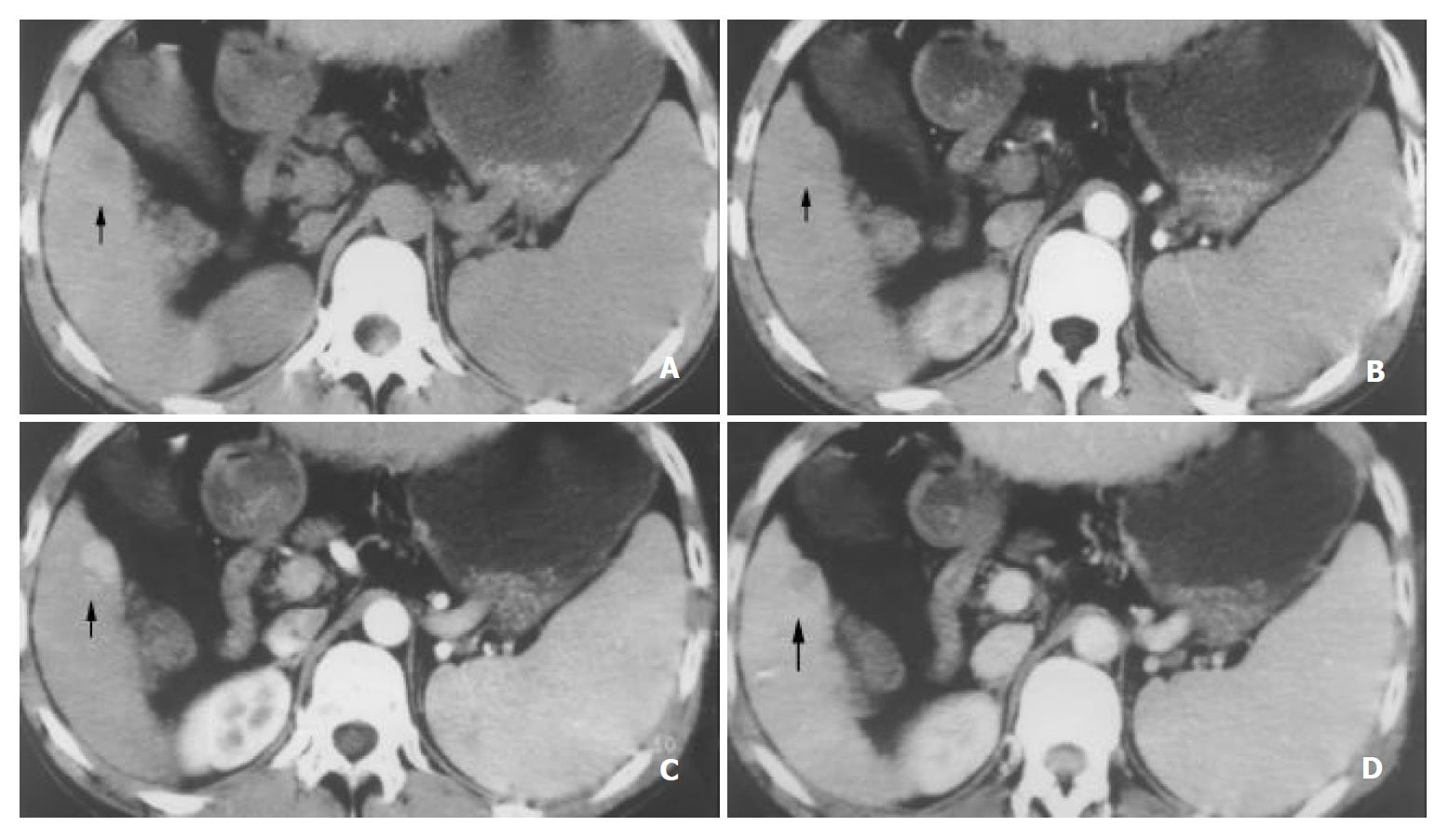
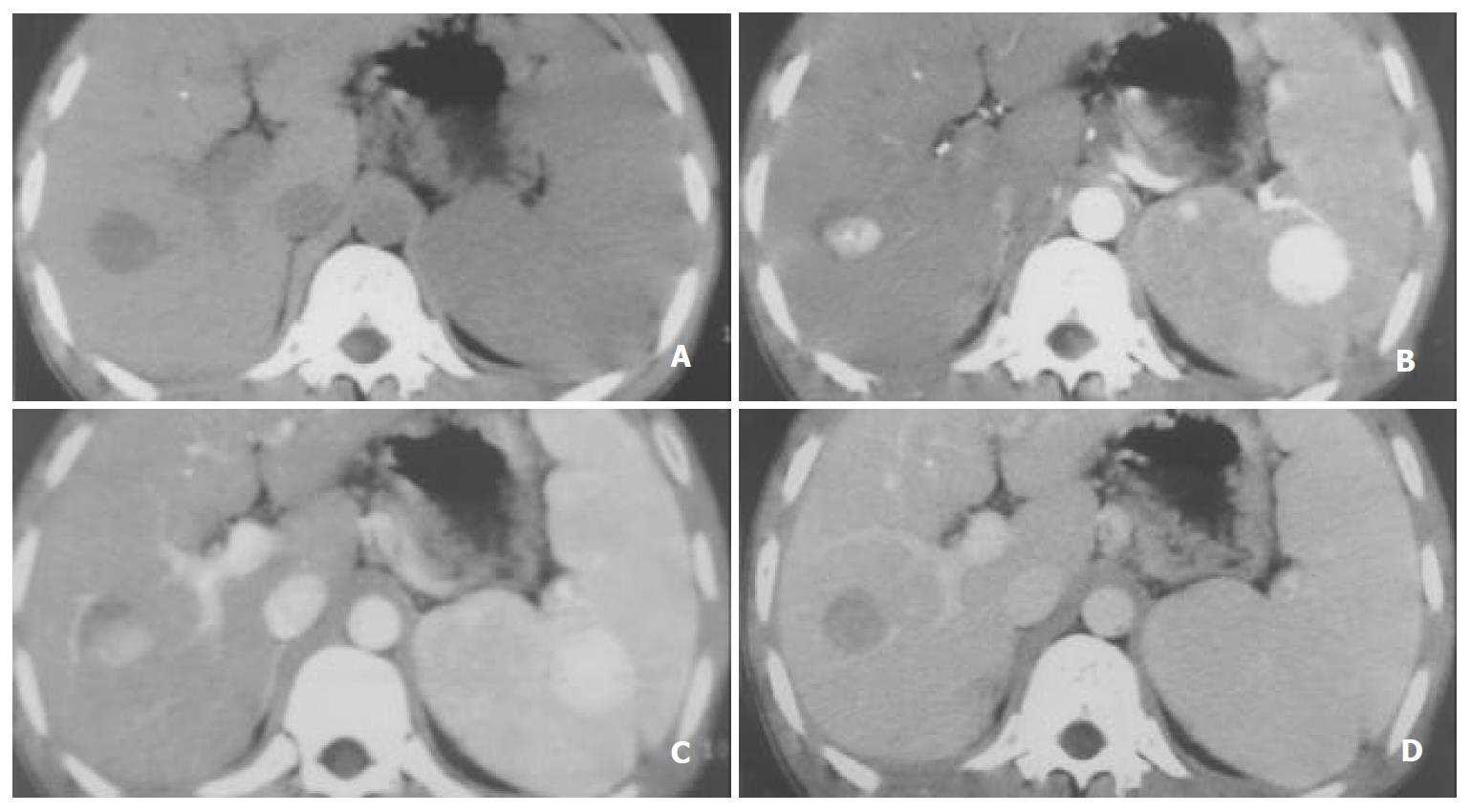
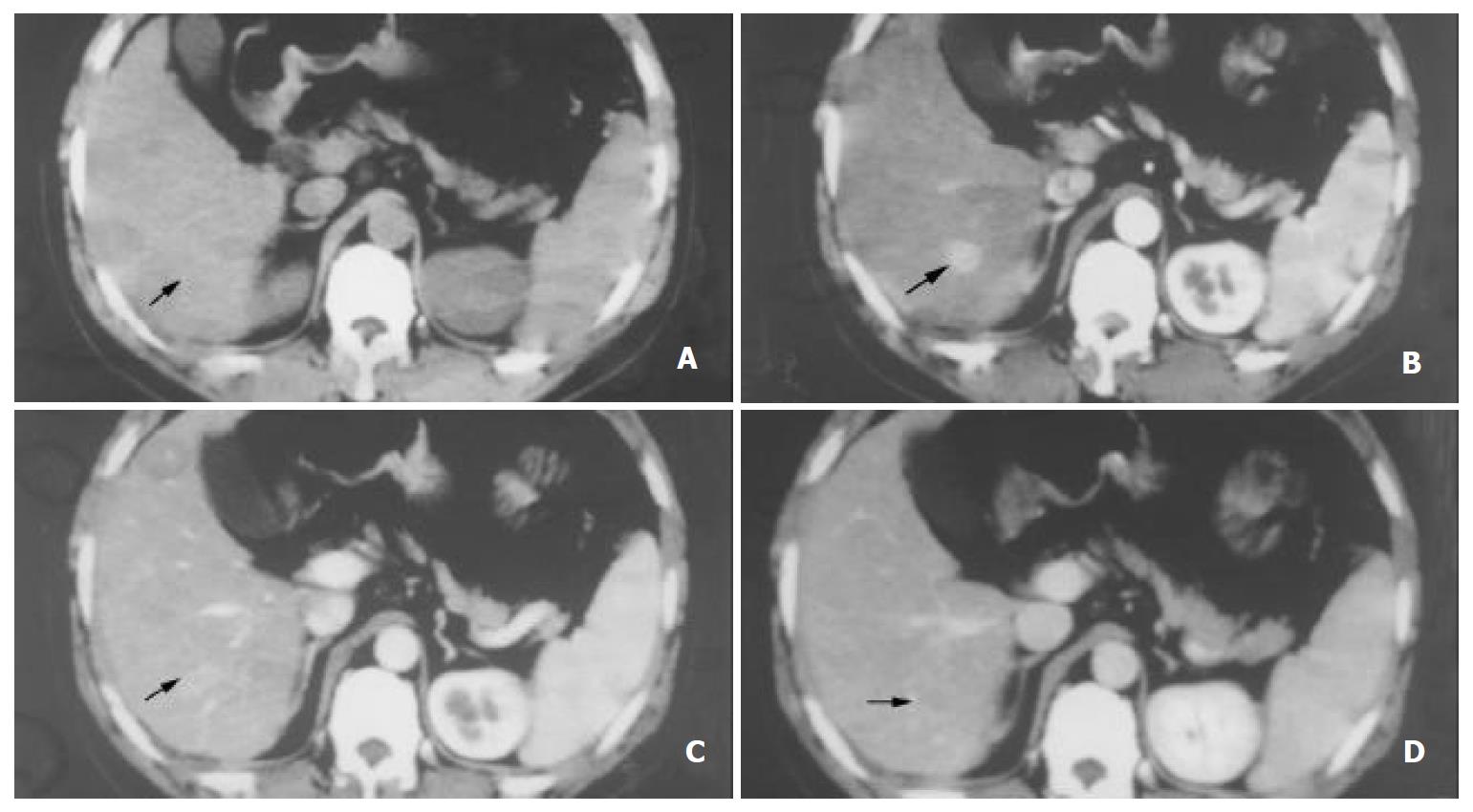
Table 2 shows that there was a significant difference in the sensitivity of SHCCs detection between EAP and LAP (45.1% vs 83.5%). Considering the importance of different phases, the sensitivity of the combined phase was 92.3%, 94.5%, and 97.8% for DAP, LAP plus PVP and DAP plus PVP, respectively, which was higher than that of each phase. (Figure 1, Figure 2, Figure 3).
The comparison of detectability is shown in Table 3. The EAP had a lower sensitivity for SHCCs compared with others (P < 0.05). A notably statistical significance was found in LAP plus PVP and EAP plus PVP (P < 0.05), but no statistical significance was observed in LAP plus PVP and DAP plus PVP.
The biphasic acquisition helical CT scanning technique has become a standard method for the detection of suspected hypervascular hepatomas. But the single-row detector helical CT (SDCT) has a slow scanning speed (0.8-1 s) and acquires only one section of CT data with each rotation of the X-ray tube. The whole liver scanning using SDCT only takes 20-25 s. It is recognized that the liver has a dual blood supply, the duration of the virtual hepatic arterial phase equals the interval from the beginning of the contrast inflow into the liver from arteries to the beginning of the contrast inflow from the portal vein. It is so short that SDCT is impossible to cover the whole liver during the real hepatic arterial phase[1-6].
Application of MDCT technology in clinical practice has brought about a decisive breakthrough, which is an important milestone in helical CT technologic revolution. The major attribution of MDCT is faster Z-axis coverage speed and improves the longitudinal resolution. MDCT can acquire multiple sections of CT data with each rotation of the X-ray tube and scan the whole liver in 4-8 s, thus imaging of the whole liver can be completed during the virtual arterial phase[7-13], even twice of the whole liver scanning can be accomplished during the virtual arterial phase. In our study the images in EAP showed intense enhancement of hepatic artery, and minimum enhancement of portal vein, and none of the hepatic parenchyma. The images in the late arterial phase demonstrated substantial portal vein, slight parenchyma enhancement, and no hepatic vein enhancement. The images in PVP showed hepatic veins enhancement, which were not yet enhanced during the early and late arterial phases[14-17].
Our previous studies[18] of continuously dynamic enhancement at the levels of hepatic artery, aorta and hepatic parenchyma, as well as at the level of SHCC foci showed that the arterial phase scanning was started at 16 s (12-22 s) and ended at 40 s. The mean duration of the arterial phase was 23 s. SHCCs were enhanced most markedly in the arterial phase. Due to its hypervascular nature , the mean peak time of the enhancement of SHCC foci was 45.4 s (31-56 s), and the maximum difference of the enhancement between tumor and liver parenchyma occurred at 36 s (28-48 s). Although SHCCs enhanced maximally at 45.4 s, the liver enhancement increased gradually and became quite obvious at that time. The conspicuity depended upon the enhancement difference of the tumor to the liver. Hence the optimally delayed scanning time should be the period when the maximum difference of enhancement occurred between tumor and liver and should not be at the peak time of the tumor enhancement[1,19,20]. This was the theoretical basis on which we set the EAP, LAP and PVP (or parenchyma phase) at 21 s, 34 s and 85 s, respectively. The delayed scanning time at different phases set up by us was in consistency with that reported by Murakami et al. They decided the delayed scanning timing for EAP (19.4 s) using a mini bolus test, and for LAP (34.9 s), just a 5 s interscan delay after the end of the first pass scanning for table increment patients’ movement and patients’ respiration[15,16].
This study showed that SHCCs were enhanced during the arterial phase gradually and declined during the portal venous phase (Figure 1). But the mean enhancement difference was greater in LAP than that in EAP (P < 0.05) (Table 1). We presume that this reflects the time interval for distribution of contrast-enhanced hepatic arterial blood into the tumor neovasculature and diffusion into the interstices of the tumor[15-22].
The results of our study showed that the sensitivity of detection of SHCC foci (n = 91) was 45.1% and 83.5% for EAP and LAP. So the late arterial phase was very important for the detection of tumor foci. During the double arterial phase, it could reach 92.3%, and was higher than that in the portal venous phase (P < 0.05). This result indicated that the arterial phase was superior to the portal venous phase in the detectability of SHCCs and was similar to that reported by other authors using biphasic scanning of SDCT[15,16]. But the CT attenuation difference of enhancement of tumors and liver between LAP and EAP led to an extremely significant difference (P < 0.001) in the detectability between LAP and EAP (83.5% vs 45.1%). Therefor, LAP is the best way for demonstrating SHCCs, which represents the advantage of triphasic scanning using MDCT. In addition, MDCT scans allow hepatic imaging with least image thickness and acquire the data in the early arterial phase, later arterial and portal venous phases and improve the sensitivity for depicting SHCC[15,16].
Since DAP combined with PVP had the highest detectability (97.8%), but with no statistical difference when compared with LAP plus PVP (94.5%). We consider the latter is more practical.
Edited by Wu XN and Wang XL
| 1. | Baron RL, Oliver JH, Dodd GD, Nalesnik M, Holbert BL, Carr B. Hepatocellular carcinoma: evaluation with biphasic, contrast-enhanced, helical CT. Radiology. 1996;199:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 258] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Oliver JH, Baron RL, Federle MP, Rockette HE. Detecting hepatocellular carcinoma: value of unenhanced or arterial phase CT imaging or both used in conjunction with conventional portal venous phase contrast-enhanced CT imaging. AJR Am J Roentgenol. 1996;167:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Oliver JH, Baron RL. Helical biphasic contrast-enhanced CT of the liver: technique, indications, interpretation, and pitfalls. Radiology. 1996;201:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Oliver JH 3rd, Baron RL, Federle MP, Jones BC, Sheng R. Hypervascular liver metastases: do unenhanced and hepatic ar-terial phase CT images effect tumor detection? Radiology. 1997;205:709-715. |
| 5. | Paulson EK, McDermott VG, Keogan MT, DeLong DM, Frederick MG, Nelson RC. Carcinoid metastases to the liver: role of triple-phase helical CT. Radiology. 1998;206:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Mitsuzaki K, Yamashita Y, Ogata I, Nishiharu T, Urata J, Takahashi M. Multiple-phase helical CT of the liver for detecting small hepatomas in patients with liver cirrhosis: contrast-injection protocol and optimal timing. AJR Am J Roentgenol. 1996;167:753-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Berland LL, Smith JK. Multidetector-array CT: once again, technology creates new opportunities. Radiology. 1998;209:327-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Lewis MA. Multislice CT: opportunities and challenges. Br J Radiol. 2001;74:779-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Spielmann AL, Nelson RC, Lowry CR, Johnson GA, Sundaramoothy G, Sheafor DH, Paulson EK. Liver: single breath-hold dynamic subtraction CT with multi-detector row helical technology feasibility study. Radiology. 2002;222:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Hu H, He HD, Foley WD, Fox SH. Four multidetector-row helical CT: image quality and volume coverage speed. Radiology. 2000;215:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 295] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Fuchs T, Kachelriess M, Kalender WA. Technical advances in multi-slice spiral CT. Eur J Radiol. 2000;36:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Hu H. Multi-slice helical CT: scan and reconstruction. Med Phys. 1999;26:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 274] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Shimizu T, Misaki T, Yamamoto K, Sueyoshi K, Narabayashi I. Helical CT of the liver with computer-assisted bolus-tracking technology: scan delay of arterial phase scanning and effect of flow rates. J Comput Assist Tomogr. 2000;24:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Sandstede JJ, Tschammler A, Beer M, Vogelsang C, Wittenberg G, Hahn D. Optimization of automatic bolus tracking for timing of the arterial phase of helical liver CT. Eur Radiol. 2001;11:1396-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Till BR, Rupert PW, Florian G. Timing of the hepatic arterial phase during contrast-enhanced computed tomography of the liver: Assessment of normal values in 25 volunteers. Invest Radiol. 2000;35:486-492. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Foley WD, Mallisee TA, Hohenwalter MD, Wilson CR, Quiroz FA, Taylor AJ. Multiphase hepatic CT with a multirow detector CT scanner. AJR Am J Roentgenol. 2000;175:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 17. | Murakami T, Kim T, Takamura M, Hori M, Takahashi S, Federle MP, Tsuda K, Osuga K, Kawata S, Nakamura H. Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology. 2001;218:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 198] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Zhou K, Yan F, Tu B. [Evaluation of the arterial phase of biphase enhanced SCT in the diagnosis of small HCC]. Zhonghua Ganzangbing Zazhi. 1999;7:135-137. [PubMed] |
| 19. | Ohashi I, Hanafusa K, Yoshida T. Small hepatocellular carcinomas: two-phase dynamic incremental CT in detection and evaluation. Radiology. 1993;189:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Hollett MD, Jeffrey RB, Nino-Murcia M, Jorgensen MJ, Harris DP. Dual-phase helical CT of the liver: value of arterial phase scans in the detection of small (& lt; or = 1.5 cm) malignant hepatic neoplasms. AJR Am J Roentgenol. 1995;164:879-884. [PubMed] |
| 21. | Ichikawa T, Kitamura T, Nakajima H, Sou H, Tsukamoto T, Ikenaga S, Araki T. Hypervascular hepatocellular carcinoma: can double arterial phase imaging with multidetector CT improve tumor depiction in the cirrhotic liver? AJR Am J Roentgenol. 2002;179:751-758. [PubMed] |
| 22. | Larson RE, Semelka RC, Bagley AS, Molina PL, Brown ED, Lee JK. Hypervascular malignant liver lesions: comparison of various MR imaging pulse sequences and dynamic CT. Radiology. 1994;192:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 4.0] [Reference Citation Analysis (0)] |