Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2178
Revised: July 25, 2003
Accepted: August 2, 2003
Published online: October 15, 2003
AIM: This study was designed to evaluate the clinical application of serum total sialic acid (TSA) in the diagnosis of cholangiocarcinoma (CCA).
METHODS: Serum TSA was determined by periodate-resorcinol microassay in 69 patients with CCA, 59 patients with hepatocellular carcinoma (HCC), 37 patients with cirrhosis, 61 patients with chronic hepatitis and 50 healthy blood donors.
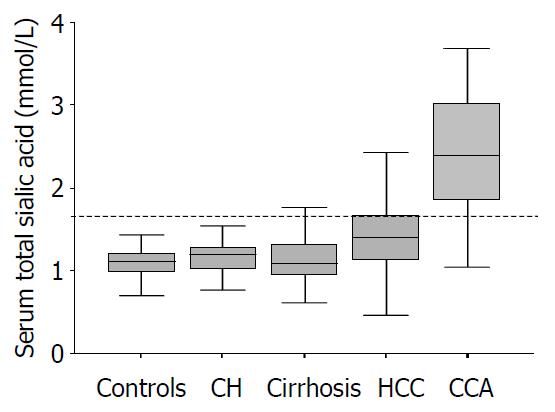
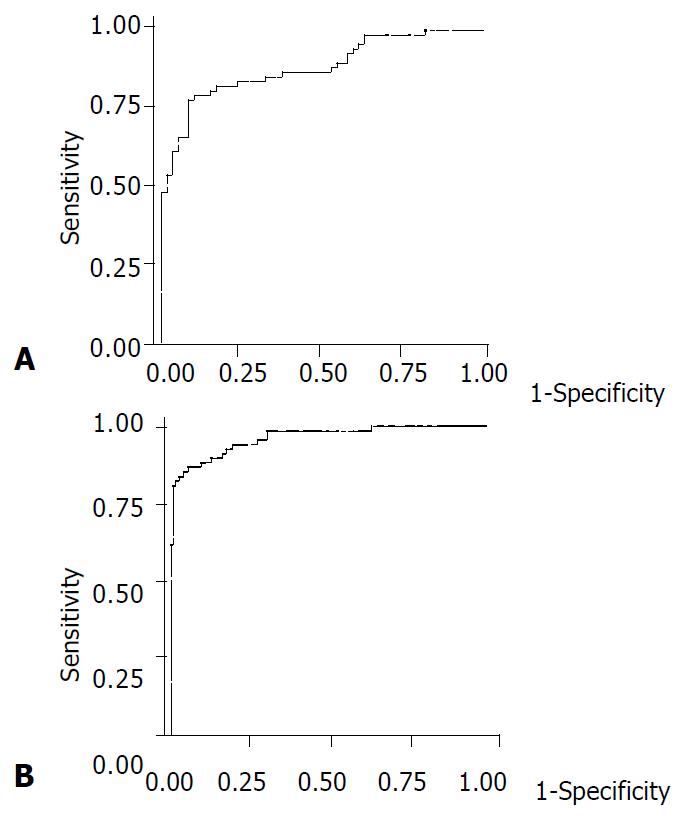
RESULTS: The mean serum TSA concentration in CCA (2.41 ± 0.70 mmol/L) was significantly higher than those of HCC, cirrhosis, chronic hepatitis and healthy blood donors (1.41 ± 0.37 mmol/L, 1.13 ± 0.31 mmol/L, 1.16 ± 0.26 mmol/L, and 1.10 ± 0.14 mmol/L, respectively; P < 0.001). Based on ROC curve analysis, a cut-off point of 1.75 mmol/L discriminated between CCA and HCC with a sensitivity, specificity and accuracy of 82.6%, 83.1%, and 82.8%, respectively.
CONCLUSION: Based on our results, serum TSA would be a useful marker for the differential diagnosis of CCA from HCC.
- Citation: Kongtawelert P, Tangkijvanich P, Ong-Chai S, Poovorawan Y. Role of serum total sialic acid in differentiating cholangiocarcinoma from hepatocellular carcinoma. World J Gastroenterol 2003; 9(10): 2178-2181
- URL: https://www.wjgnet.com/1007-9327/full/v9/i10/2178.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i10.2178
Cholangiocarcinoma (CCA) constitutes a common primary liver cancer in Southeast Asia where the liver fluke, Opisthorchis viverrini, is endemic[1]. Most patients with CCA are diagnosed at advanced stages, therefore, treatment of the cancer is usually palliative and the prognosis is poor[2]. Currently, there is no ‘gold standard’ tumor marker for the diagnosis of CCA. This is particularly remarkable for early detection of the tumor itself, for screening of the high-risk groups, and for differentiating CCA from hepatocellular carcinoma (HCC), another primary liver cancer which is common in Southeast Asia and frequently associated with chronic hepatitis B or C[3]. Among the available serum tumor markers, the most commonly used is a high-molecular-weight glycolipid, carbohydrate antigen 19-9 (CA 19-9). CA 19-9, however, is not a sensitive or specific tumor marker for CCA. As a single diagnostic test, CA 19-9 increases in approximately 65% of liver fluke-associated CCA[4]. Elevated concentrations of this marker have also been observed in patients with a variety of gastrointestinal cancers, as well as benign cholestasis and acute cholangitis[5]. As a result, a more sensitive and specific serum marker for the diagnosis of CCA is considered necessary.
Sialic acid, a class of important ketoses that contain nine carbon atoms, is an acetylated derivative of neuraminic acid (2-keto-5-amino-3, 5-dideoxy-D-nonulosonic acid)[6]. The unique structural features of this molecule, which includes a negative charge owing to a carboxyl group, enable it to play an important role in cellular functions, such as cell-to-cell recognition and transformation to malignancy[7]. Elevated levels of serum total sialic acid (TSA) have been reported in patients diagnosed with various cancers such as lymphoma, malignant melanoma, lung cancer and gastrointestinal cancers[8,9]. Recently, it has been shown that most patients with CCA have an elevated concentration of serum TSA, and determination of this marker yields high diagnostic values that differentiate between CCA and benign hepatobiliary diseases[10]. However, the diagnostic role of the serum marker in discriminating CCA from HCC has never been verified.
Therefore, the aim of this study was to use a simple technique (microassay) to determine the clinical application of serum TSA in the diagnosis of CCA by comparison with HCC and other chronic liver diseases including chronic hepatitis and cirrhosis.
Sera for the measurement of TSA levels were obtained from 5 groups of subjects who were attending King Chulalongkorn Memorial Hospital and Udonthani Hospital from January 1998 to July 1999.
Group 1 consisted of 50 adult healthy blood donors as control subjects.
Group 2 consisted of 61 patients with chronic hepatitis which was diagnosed based on histopathology.
Group 3 consisted of 37 patients with cirrhosis. The diagnosis of cirrhosis was based on histopathology and/or clinical features such as the presence of ascites, or esophageal varices.
Group 4 consisted of 59 patients with HCC. The diagnosis of HCC was based on histopathology and/or imaging techniques combined with serum alpha-fetoprotein levels above 400 ng/mL.
Group 5 comprised 69 patients with CCA. All patients in this group were residents of Thailand’s northeastern provinces where O. viverrini was endemic. The peripheral type CCA was diagnosed based on liver tumor features detected by ultrasound/CT scan and confirmed by histology. Criteria for diagnosis of the hilar type included findings of primary mass at the hilum and the evidence of bile duct dilatation on ultrasound/CT scan and confirmed by characteristic features on cholangiography or histopathology.
All subjects were informed about the objective of the study, and subsequently provided their consent. Blood was obtained during investigation at the initial presentation, sera were separated by centrifugation and stored at -70 °C at Viral Hepatitis Research Unit until tested for TSA concentrations.
Serum TSA determination was performed as previously described[6], with some modifications. Briefly, 40 μL of samples or pure standard sialic acid solution (2-10 μg/well) was added to the wells of a 96-well microtiter plate. Then, 50 μL of 1.3 mM periodic acid (prepared from stock 0.32 M), was added to each well and mixed by shaking the plate for 5 min on a microplate shaker at room temperature. The plate was placed (floated) in an icebox for 60 min, then 100 μL of 0.6 g/dL of resorcinol reagent (prepared from stock 6 g/dL) was added and mixed by shaking as described above. The plate was covered with a glass and heated at 80 °C for 60 min in water bath, then it was removed and placed on the shaker for about 2 min mixing as well as cooling the contents down to room temperature. Then, 100 μL of 95% tert-butyl alcohol was added to each well and mixed once again as described above. The absorbance at 620 nm was measured immediately by a microtiter plate reader.
The precision of the test was determined by analysis of an intra- and inter-assay coefficient variation (CV). The method described herein demonstrated an intra- and inter-assay CV of 0.79% and 4.68%, respectively. Furthermore, the recovery percentage of this assay was 94.25%.
The data were expressed as mean values ± standard deviation. Statistical significance in the mean values was evaluated by the Student’s t test. Receiver-operating characteristic (ROC) curves were constructed to establish the diagnostic cut-off level of serum TSA in discriminating CCA from other groups. Sensitivity, specificity, positive and negative predictive values and diagnostic accuracy were calculated in accordance with standard methods. P value below 0.05 was considered statistically significant.
Comparison of the characteristics of the subjects in each group (Table 1) showed that the mean age of patients with CCA (60.2 ± 12.6 years) was significantly higher than those with HCC and cirrhosis (53.0 ± 12.0 and 50.0 ± 13.3 years, respectively; P = 0.001), chronic hepatitis and blood donors (42.0 ± 13.0 and 34.6 ± 10.3 years, respectively; P < 0.001). There was no significant difference in the sex distribution in each group. Mean total bilirubin level was significantly higher in patients with CCA compared with those with HCC, cirrhosis and chronic hepatitis (P < 0.05), whereas mean serum alkaline phosphatase was significantly higher in patients with CCA compared to those with cirrhosis and chronic hepatitis (P < 0.05).
| Group | No | Age (yr) | Sex (M/F) | TB (mg/dl) | ALT (IU/L) | Alb (g/l) | AP (IU/L) |
| Controls | 50 | 34.6 ± 10.3a | 29/21 | - | - | - | - |
| CH | 61 | 42.0 ± 13.0a | 46/15 | 1.6 ± 0.9a | 98.1 ± 72.9 | 3.8 ± 0.3 | 201.5 ± 106.2a |
| Cirrhosis | 37 | 50.0 ± 13.3a | 26/11 | 2.5 ± 1.3a | 65.0 ± 49.6 | 3.3 ± 0.5 | 290.2 ± 212.5a |
| HCC | 59 | 53.0 ± 12.0a | 50/9 | 3.0 ± 2.1a | 79.9 ± 46.1 | 3.4 ± 0.3 | 612.9 ± 393.7 |
| CCA | 69 | 60.2 ± 12.6 | 46/23 | 7.5 ± 7.6 | 71.4 ± 68.7 | 3.4 ± 0.5 | 894.3 ± 850.2 |
The distributions of serum TSA levels in each group are shown in Figure 1. The mean serum TSA concentration in patients with CCA (2.41 ± 0.70 mmol/L) was significantly higher than that in those with HCC, cirrhosis, chronic hepatitis and blood donors (1.41 ± 0.37 mmol/L, 1.13 ± 0.31 mmol/L, 1.16 ± 0.26 mmol/L, and 1.10 ± 0.14 mmol/L, respectively; P < 0.001). The mean concentration of serum TSA in patients with HCC was also significantly higher than that in those with cirrhosis, chronic hepatitis and blood donors (P = 0.001). However, there was no significant difference in mean serum level of TSA among patients with cirrhosis, chronic hepatitis and blood donors.
In order to discriminate CCA from HCC with an optimal accuracy, an analysis of the ROC curve was performed. As shown in Figure 2A, the area under the curve of CCA and HCC was 0.885 [95% confidence interval (CI) 0.828-0.0.942]. This result indicated that approximately 89% of randomly selected patients from the positive group (CCA) would have a higher TSA value than a patient randomly selected from the HCC group. Similarly, the area under the ROC curve of CCA and the other three groups (cirrhosis, chronic hepatitis and healthy controls) was 0.964 (95%CI 0.938-0.0.989) (Figure 2B), indicating that approximately 96% of patients with CCA would have a higher level of serum TSA than patients with cirrhosis, chronic hepatitis and healthy controls.
Based on the ROC curve analysis, a cut-off point of serum TSA concentration considered as the highest accuracy for diagnosing CCA was 1.75 mmol/L. At this concentration, the sensitivity, specificity and accuracy for differentiating CCA from HCC were 82.6%, 83.1%, and 82.8%, respectively. Likewise, when compared to patients with cirrhosis, chronic hepatitis and healthy blood donors, the same cut-off level of serum TSA exhibited a sensitivity, specificity and accuracy for diagnosing CCA of 82.6%, 86.0%, and 84.8%, respectively.
Liver fluke-associated CCA is typically associated with its insidious onset of clinical manifestations, which frequently escapes detection until late stages. The clinical features of CCA appeared to differ according to the location of the tumor along the intrahepatic duct, whether in the hilum or periphery[11,12]. The hilar type tends to present with progressive jaundice or ascending cholangitis due to tumor obstructtion in the confluence of the hepatic ducts, while the peripheral type usually presents with weight loss and progressive right upper quadrant pain caused by the expanding tumors. In areas where both liver flukes and chronic viral hepatitis are prevalent, it remains an essential problem to differentiate CCA from HCC. Although HCC is usually associated with cirrhosis, while conversely, CCA develops in a non-cirrhotic liver, the occurrence of CCA in cirrhosis has been increasingly recognized[13]. Moreover, approximately 10% of patients with HCC presented with obstructive jaundice, a clinical feature that might imitate the hilar type of CCA[14]. Accordingly, a sensitive and specific serum marker of CCA would be considered as a valuable adjunct to non-invasive imaging for the diagnosis of the cancer.
Serum TSA has been reported as a diagnostic maker for patients with various cancers such as lymphoma, malignant melanoma, lung cancer and gastrointestinal cancers[8,9]. A variety of methods for the detection and estimation of free and glycosidically-bound sialic acids have been performed. These techniques could be broadly classified as colorimetric, fluorometric, enzymatic methods, as well as the highly sensitive high performance liquid chromatographic (HPLC) method[7]. Among these, the most widely used technique is the colorimetric method, which includes the Ehrlich method, the periodate-thiobabituric acid method, and the periodic-resorcinol assay. In this report, we employed a newly developed periodate-resorcinol micorassay that has several advantages over the conventional methods. These include use of smaller samples (5 μL), a larger number of samples simultaneously analyzed, and a greater speed in measuring absorbance by a microtiter plate spectrophotometer. Moreover, this method enables the direct transfer of data to a computer, or even an adaptation to an automated system.
In this study, we demonstrated that increased serum TSA concentration yielded a high sensitivity, specificity and accuracy for the diagnosis of CCA. Our data showed that the mean serum TSA level in patients with CCA was significantly increased compared to those with cirrhosis, chronic hepatitis and healthy controls (P < 0.001). Notably, the mean concentration of serum TSA in patients with CCA was substantially higher than that in those with HCC (P < 0.001). Based on the ROC curve analysis, a cut-off point of 1.75 mmol/L provided a satisfactorily high sensitivity, specificity and accuracy of above 80% for the differential diagnosis of CCA from HCC. The data of the present study are in agreement with those previously reported which described an increase in serum TSA in patients with CCA[10,15]. Thus, serum TSA would be a useful marker, particularly in conjunction with radiological studies such as ultrasonography or CT scan, for the detection of CCA in patients presenting with clinical features of liver mass and jaundice.
The mechanisms underlying the substantial difference in levels of TSA between patients with CCA and HCC are unclear. One possible explanation is that, in certain cancers, increased activity of sialytransferase might lead to spontaneous shedding of aberrant sialic acid-containing cell surface glycoconjugates into the circulation[16]. Alternatively, since TSA has been well described as being associated with the acute-phase protein response[17], increased activity of serum or tissue sialidase in combination with inflammatory response could considerably elevate serum TSA levels in patients with CCA. Also, the difference between the ages of the CCA and HCC groups might be responsible for this discrepancy, since serum TSA concentration slightly increased with advancing age[18]. Nonetheless, the age-dependent difference between groups might not be sufficient to interfere with the discrimination of serum TSA values in our study. To confirm this observation, the group of age-matched patients with HCC should also be examined for serum TSA levels.
In conclusion, we have demonstrated that serum TSA concentrations, which were determined by a simple microassay, are significantly higher in patients with CCA than in those with HCC, cirrhosis, chronic hepatitis and blood donors. At a cut-off point of 1.75 mmol/L, this serum marker can yield a satisfactory accuracy for the differential diagnosis of CCA from HCC. Accordingly, the measurement of serum TSA level might be useful for the diagnosis of CCA, particularly in the regions where liver flukes are common.
We would like to thank Venerable Dr. Mettanando Bhikkhu of Wat Nakprok, Bangkok, for editing the manuscript. This work was supported by grants from the National Research Council, Bangkok, the Center of Excellent Fund, Center of Excellence Viral Hepatitis Research Unit, Faculty of Medicine, Chulalongkorn University, the Thailand Research Fund, Senior Research Scholar (YP) and Junior Research Scholar (PK).
Edited by Wang XL
| 1. | Parkin DM, Srivatanakul P, Khlat M, Chenvidhya D, Chotiwan P, Insiripong S, L'Abbe KA, Wild CP. Liver cancer in Thailand. I. A case-control study of cholangiocarcinoma. Int J Cancer. 1991;48:323-328. [RCA] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Watanapa P. Cholangiocarcinoma in patients with opisthorchiasis. Br J Surg. 1996;83:1062-1064. [PubMed] |
| 3. | Tangkijvanich P, Hirsch P, Theamboonlers A, Nuchprayoon I, Poovorawan Y. Association of hepatitis viruses with hepatocellular carcinoma in Thailand. J Gastroenterol. 1999;34:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Pungpak S, Akai PS, Longenecker BM, Ho M, Befus AD, Bunnag D. Tumour markers in the detection of opisthorchiasis-associated cholangiocarcinoma. Trans R Soc Trop Med Hyg. 1991;85:277-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Duffy MJ. CA 19-9 as a marker for gastrointestinal cancers: a review. Ann Clin Biochem. 1998;35:364-370. [PubMed] |
| 6. | Bhavanandan VP, Sheykhnazari M. Adaptation of the periodate-resorcinol method for determination of sialic acids to a microassay using microtiter plate reader. Anal Biochem. 1993;213:438-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Narayanan S. Sialic acid as a tumor marker. Ann Clin Lab Sci. 1994;24:376-384. [PubMed] |
| 8. | Kökoğlu E, Sönmez H, Uslu E, Uslu I. Sialic acid levels in various types of cancer. Cancer Biochem Biophys. 1992;13:57-64. [PubMed] |
| 9. | Polívková J, Vosmiková K, Horák L. Utilization of determining lipid-bound sialic acid for the diagnosis and further prognosis of cancer. Neoplasma. 1992;39:233-236. [PubMed] |
| 10. | Wongkham S, Boonla C, Kongkham S, Wongkham C, Bhudhisawasdi V, Sripa B. Serum total sialic acid in cholangiocarcinoma patients: an ROC curve analysis. Clin Biochem. 2001;34:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Uttaravichien T, Buddhisawasdi V. Experience of non-jaundiced cholangiocarcinoma. Hepatogastroenterology. 1990;37:608-611. [PubMed] |
| 12. | Kullavanijaya P, Tangkijvanich P, Poovorawan Y. Current status of infection-related gastrointestinal and hepatobiliary diseases in Thailand. Southeast Asian J Trop Med Public Health. 1999;30:96-105. [PubMed] |
| 13. | Hui CK, Yuen MF, Tso WK, Ng IO, Chan AO, Lai CL. Cholangiocarcinoma in liver cirrhosis. J Gastroenterol Hepatol. 2003;18:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Qin LX, Tang ZY. Hepatocellular carcinoma with obstructive jaundice: diagnosis, treatment and prognosis. World J Gastroenterol. 2003;9:385-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Wongkham S, Bhudhisawasdi V, Chau-in S, Boonla C, Muisuk K, Kongkham S, Wongkham C, Boonsiri P, Thuwajit P. Clinical significance of serum total sialic acid in cholangiocarcinoma. Clin Chim Acta. 2003;327:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Singhal A, Hakomori S. Molecular changes in carbohydrate antigens associated with cancer. Bioessays. 1990;12:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Crook MA, Treloar A, Haq M, Tutt P. Serum total sialic acid and acute phase proteins in elderly subjects. Eur J Clin Chem Clin Biochem. 1994;32:745-747. [PubMed] |
| 18. | Stefenelli N, Klotz H, Engel A, Bauer P. Serum sialic acid in malignant tumors, bacterial infections, and chronic liver diseases. J Cancer Res Clin Oncol. 1985;109:55-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 1.9] [Reference Citation Analysis (0)] |