INTRODUCTION
Invasion and metastasis are malignant characteristies of HCC and the main mortal reason for patients[1,2]. In the invasive and metastatic process of malignant tumor, molecules existing in extracellular matrix (ECM) and receptors or ligands existing on the surfaces of tumor cells play critical roles[3,4]. HAb18G is such a molecule obtained by screening cDNA library with HAb18 specific monoclonal antibody (mAb) against HCC[5-7]. The gene sequence of HAb18G is homologous to that of CD147. CD147, also named extracelluar matrix metalloproteinase inducer (EMMPRIN), is originated from human lung cancer cell line LX-1, firstly found in human HCC tissue in our laboratory. Previous studies demonstrated that EMMPRIN, a member of the immunoglobulin superfamily, concentrated on the surfaces of most tumor cells, promoted invasion of tumor cells by stimulating stromal cells to produce elevated levels of several matrix metalloproteinase (MMPs)[8,9] which play very important roles in several aspects of tumor progression, including growth, invasion, metastasis, and angiogenesis[10-12]. To study the relationship between HAb18G/CD147 and metastasis of HCC, we constructed a vector of antisense RNA of HAb18G/CD147 and investigated its inhibitory effects on invasion of HCC cells in vitro.
MATERIALS AND METHODS
Plasmid, bacteria strain and cell line
Plasmid pBluescript ks (+/-)/HAb18G including full length cDNA of HAb18G/CD147 was constructed by our laboratory. Eukaryotic expressing vector PCI-neo was purchased from Promega. E.Coli strain JM109 was purchased from Huamei Co, Ltd. Human HCC cell line HHCC was purchased from Type Culture Collection of Chinese Academy of Sciences, Shanghai, China. Human embryo dermal fibroblast cell line (fb) was kindly presented by Dr. Han (Department of Plastic Surgery, Fourth Military Medical University).
Construction and identification of antisense vector
Full length cDNA fragment of HAb18G/CD147 was obtained from pBluescript ks (+/-)/HAb18G by cutting with Xba I and Xho I and inserted reversely into eukaryotic expressing vector PCI-neo by cutting with the same restriction endonucleases. The antisense vector of HAb18G/CD147, named as PCI-asHAb18G, was transformed into E.coli stain JM109 and identified by restricted endonuclease digestion and DNA sequences analysis by an automatic fluorescence sequenator using T3 sequencing primer.
Cloning of cell transfected with antisense RNA vector
Empty vector PCI-neo and antisense vector PCI-asHAb18G were transfected into human HCC cell line HHCC via cation liposome Lipfectinamine2000 (Gibco) respectively. The two kinds of transfected cells were named as HHCC/neo and HHCC/asHAb18G respectively. All transfection procedures were performed according to the product manual. After two days of transfection, cells were selected by culture medium containing G418 (400 mg/L) for 4 weeks. The single clone was picked out by using limiting dilution method and cultured constantly in the culture medium containing G418 (100 mg/L).
Immunohistochemical staining of transfected cells
Transfected cells were plated on glass slides overnight, fixed with cold acetone, and stained with SP (streptomycin avidin-peroxidase) according to the manufacturer’s instructions. Briefly, mAb HAb18 was used as primary antibody and the goat anti-mouse mAb coupled with biotin as secondary antibody followed by indirect immunohistochemical staining with the mixture of streptomycin-avidin peroxidase and its substrate DAB. HHCC/neo served as the control.
FACS analysis
HHCC/asHAb18G suspension was prepared by adding primary antibody and secondary antibody coupled with fluorescein into 108 cells per liter. Then the cells were fixed and analyzed by flow cytometer.
Gelatin zymography
Five experimental groups of cells were HHCC, fb, HHCC/neo + fb, HHCC/asHb18G + fb and HHCC + fb, which were plated into 100 mL culture flasks respectively. The ratio of HCC cells/fb cells was 1:1. After cultured in completed DMEM medium for 24 h, all the groups of cells were washed three times with serum free DMEM and cultured for 2-3 d in DMEM with 20 mL/L bovine serum. Subsequently, the supernatants were collected and centrifuged to remove the cell debris. Proteins were precipitated with 800 g/L saturated (NH4)2SO4. Precipitations were dissolved in 10 mmol/L Tris-HCl, pH7.5 and dialyzed. Dialyzed samples were determined with SDS-PAGE that was modified in four points. Gelatin (Sigma) was added into the separating gel with 1.0 g/L, concentration of the stacking gel was 5%, samples were not boiled and sample buffers did not contain DTT. After electrophoresis, the gel was washed with 0.1 mol/L NaCl and incubated for 24 h at 37 °C. Finally, the gel was dyed and decolorized.
Reconstituted basement membrane invasion detection
Matrigel (main component was type IV collagen, purchased from Cell-biology Department of Pecking University) was added onto the inner surface of Boyden chambers (Millipore) to form the reconstituted basement membrane. Three groups of cells were HHCC + fb, HHCC/asHAb18G + fb, HHCC/neo + fb (the ratio of HCC cells/fb cells was 1:1), which were added on the reconstituted basement membrane respectively. Then the chambers were put in the 24-well plates and cultured overnight. Cells infiltrated through the reconstituted basement membrane and appeared on the outer surfaces of the membrane were stained with HE. The numbers of the cells were counted under high-power microscope.
RESULTS
Construction and identification of PCI-asHAb18G
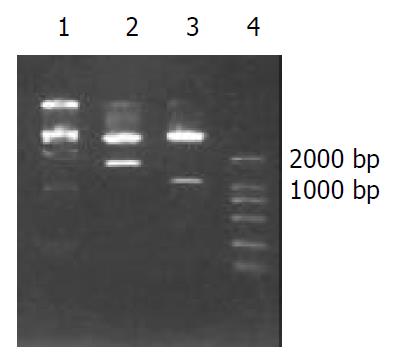
Two fragments were obtained by digesting HAb18G/CD147 antisense vector with Xho I and Xba I , one was human HAb18G/CD147 cDNA fragment about 1.7 kb and the other was 5.5 kb fragment. Two fragments of 1.1 kb and 6.1 kb were also obtained with SmaI digestion (both MCS of vector and 1.1 kb site of HAb18G/CD147 cDNA respectively have a SmaI site). The construction of the vector was verified correct by endonuclease digestion (Figure 1) and nucleotide sequencing. The vector was named as PCI-asHAb18G.
Figure 1 Restricted endonuclease analysis of recombinant plas-mid PCI-asHAb18G.
1: DNA marker DL15000, 2: PCI-asHAb18G/Xho I + Xba I, 3: PCI-asHAb18G/SmaI, 4: DNA marker DL2000.
Immunohistochemical staining and FACS
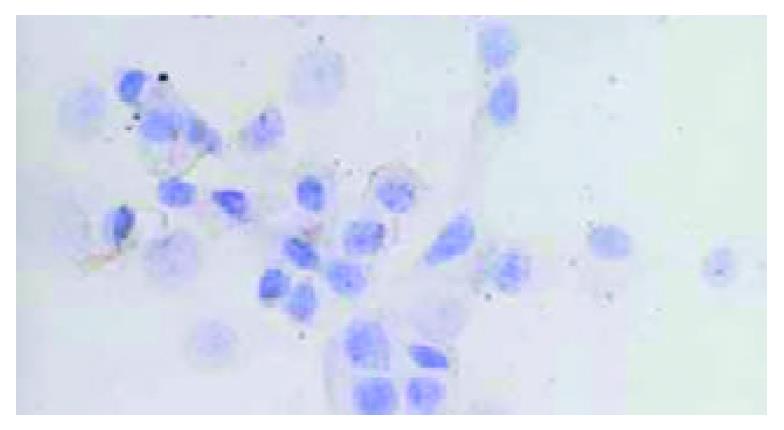
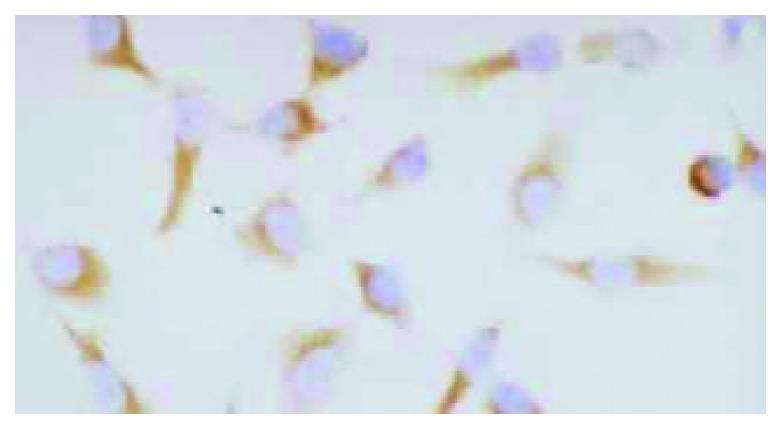
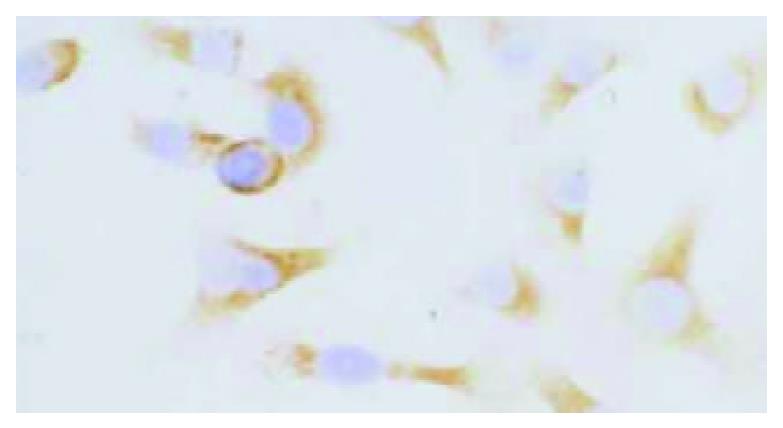
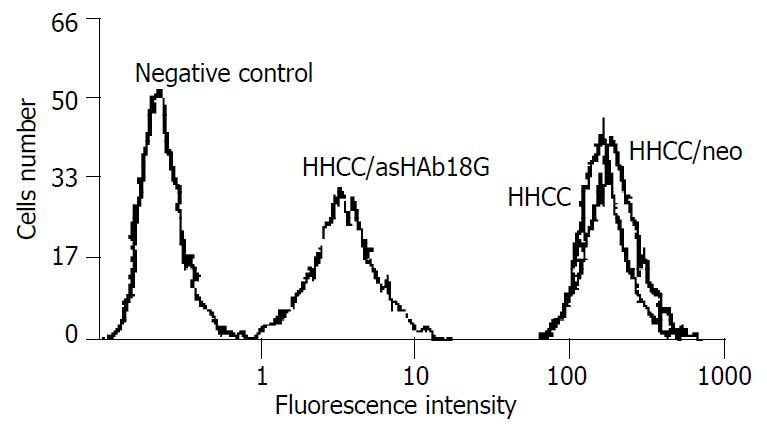
HHCC/as HAb18G was negative, HHCC/neo and HHCC groups were positive (Figure 2, Figure 3, Figure 4). Average value of the fluorescence intensities of HHCC/asHAb18G cells was 5, and that of HHCC/neo cells was 500 (Figure 5).
Figure 2 Negative staining of HAb18G/CD147 on membrane of HHCC/asHAb18G SP × 400.
Figure 3 Positive staining of HAb18G/CD147 on membrane of HHCC/neo SP × 400.
Figure 4 Positive staining of HAb18G/CD147 on membrane of HHCC SP × 400.
Figure 5 FACS analysis of expressions of HAb18G/CD147 in four kinds of cells.
Gelatin zymography and cell invasion
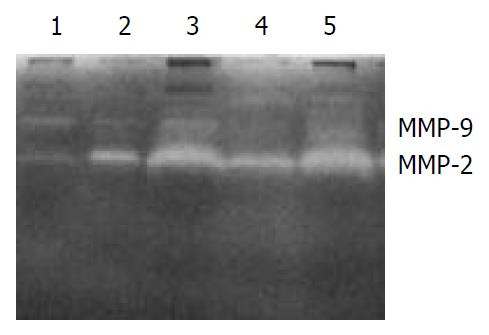
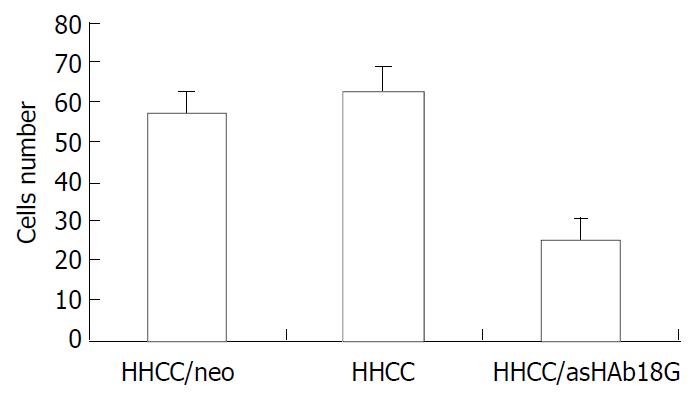
The secretions of MMP-9 and MMP-2 in the transfected cells HHCC/asHAb18G co-cultured with fb were inhibited compared to those in the HHCC/neo and intact HHCC. The cells of HHCC/asHAb18G infiltrated through the reconstituted basement membrane were less than those of HHCC/neo and intact HHCC (Figure 6, Figure 7).
Figure 6 Gelatin zymography of secretions of MMP-2 and MMP-9 in five groups of cells.
1: fb, 2: HHCC, 3: HHCC/neo + fb, 4: HHCC/asHAb18G + fb, 5: HHCC + fb.
Figure 7 Inhibitory effects of antisense RNA on invasion of HHCC/asHAb18G.
DISCUSSION
Matrix metalloproteinases (MMPs) play an important part in tumor progression and tumor cell survival, with a positive correlation between MMP expression and the invasive and metastatic potential of malignant tumors, including colon, lung, head and neck, basal cell, breast, thyroid, prostate, ovarian, and gastric carcinomas. CD147 is a heavily glycosylated transmembrane glycoprotein containing two immunoglobulin superfamily domains, which induces MMPs production in the adjacent stromal cells. Some results implied that a CD147 counter-receptor might existed on the fb cell surface, but such a counter- receptor has not been identified[13]. Other studies[14] showed that CD147 also acted in an autocrine fashion to increase productions of MMPs and invasiveness in tumor cells themselves. CD147 enriched in HCC tissue was first reported by our laboratory and might be a potential target for anti-invasion and metastasis therapies. Our previous studies showed that HAb18G/CD147 was highly expressed in HCC tissues and lowly expressed in normal tissues. HAb18G/CD147, not only participated adhesion of cell-cell or cell-matrix but also enhanced metastatic potentials of human hepatoma cells by disrupting the regulation of store-operated Ca (2+) entry by NO/cGMP[15,16].
The principle of antisense technology is the sequence-specific binding of an antisense oligonucleotide to target mRNA, resulting in the prevention of gene translation. The specificity of hybridisation makes antisense treatment an attractive strategy selectively modulating the expression of genes involved in the pathogenesis of diseases. In 1998, the first antisense drug (fomivirsen) was approved by the US Food and Drugs Administration (FDA) for the treatment of cytomegalovirus-induced retinitis in patients with AIDS[17]. Now, several antisense oligonucleotides have been under clinical trials, including oligonucleotides targeting the mRNA of Bcl-2[18,19], protein-kinase-C alpha[20,21], RAF kinase[22,23], H-ras[24,25], C-myb[26], DNA methyltransferase[27] and RI-alpha regulatory subunit of protein kinase A (PKA)[28,29]. Antisense oligonucleotides are well tolerated and might have therapeutic activities.
Our experimental findings showed that the expression of HAb18G/CD147 on HCC cells transfected by PCI-asHAb18G was decreased by analysis of immunohistochemical staining and FACS. Gelatin zymography demonstrated that secretions of MMP-2 and MMP-9 of HHCC/asHAb18G were lower than those of HHCC/neo and HHCC. Matrigel invasion assay indicated that invasion of HHCC/asHAb18G cells was inhibited significantly. All of the results confirm that antisense RNA targeting HAb18G/CD147 mRNA interfered with translation and expression of HAb18G/CD147, weakened the productions of MMPs, and inhibited the invasion of HCC cells through reconstituted basement membrne in vitro.
We suggest that HAb18G/CD147 be used as a novel target for anti-hepatoma metastatic therapy. Intervention in HAb18G/CD147 function by agents, such as antisense RNA, may have potential therapeutic value in the prevention of hepatoma invasion and metastasis.