Published online Jan 15, 2003. doi: 10.3748/wjg.v9.i1.174
Revised: July 25, 2002
Accepted: July 27, 2002
Published online: January 15, 2003
AIM: With successful surgical treatment of gastroesophageal reflux disease (GERD), there is interest in understanding the anti-reflux barrier and its mechanisms of failure. To date, the potential use of vector volumes to predict the DeMeester score has not been adequately explored.
METHODS: 627 patients in the referral database received esophageal manometry and ambulatory 24-hour pHmonitoring. Study data included LES resting pressure (LESP), overall LES length (OL) and abdominal length (AL), total vector volume (TVV) and intrabdominal vector volume (IVV).
RESULTS: In cases where LESP, TVV or IVV were all below normal, there was an 81.4% probability of a positive DeMeester score. In cases where all three were normal, there was an 86.9% probability that the DeMeester score would be negative. Receiver-operating characteristics (ROC) for LESP, TVV and IVV were nearly identical and indicated no useful cut-off values. Logistic regression demonstrated that LESP and IVV had the strongest association with a positive DeMeester score; however, the regression formula was only 76.1% accurate.
CONCLUSION: While the indices based on TVV, IVV and LESP are more sensitive and specific, respectively, than any single measurement, the measurement of vector volumes does not add significantly to the diagnosis of GERD.
- Citation: Marsh RE, Perdue CL, Awad ZT, Watson P, Selima M, Davis RE, Filipi CJ. Is analysis of lower esophageal sphincter vector volumes of value in diagnosing gastroesophageal reflux disease? World J Gastroenterol 2003; 9(1): 174-178
- URL: https://www.wjgnet.com/1007-9327/full/v9/i1/174.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i1.174
With the recognition of gastroesophageal reflux disease (GERD) as a surgical pathology, there is deep interest in understanding the anatomical and physiological anti-reflux barrier and its mechanisms of failure[1-8] The anatomical components of the barrier include the crural fibers of the diaphragmatic esophageal hiatus, the smooth muscle sling fibers of the gastric cardia, and the semicircular and clasp fibers of the distal esophagus[1-5]. Augmented by positive intrabdominal pressure over the most distal portion of the lower esophagus and proximal cardia, the sphincter approximates the mucosal epithelium covering the internal surface area of the esophagogastric junction. This distinct high-pressure zone is a critical factor in the barrier against reflux of gastric contents[5-8]. The anatomical function of the lower esophageal sphincter (LES) is complemented by neutralization of refluxate by alkaline oral secretions and rapid clearance of esophageal contents by intermittent and reflex peristalsis[8].
Various means have been devised to measure the mechanical integrity of the LES[8-11]. Traditional pull-through manometry has been succeeded in some laboratories by sophisticated equipment and software that allows measurement of the closure pressure generated by the three-dimensional sphincter mechanism. Directional pressures can be summated over the length of the sphincter to produce a vector volume that describes the overall physical resistance of the barrier to continuous reflux[11,12]. Other measures of the sphincter competence include resting pressure of the LES at various points along its length, the vector volume of the intrabdominal portion of the LES as well as overall and intrabdominal lengths of the LES. While many observers have noted the relationship between abnormal manometry and GERD[4-14], we have sought to quantify this relationship in a way that describes the comparative ability of such measurements to predict positive ambulatory pHmonitoring as described by DeMeester and colleagues[15]. It was our goal to compare traditional manometric measurements, vector volume analysis and results of 24-hour pHmonitoring. We postulated that one or more of the manometric measurements would yield a statistically significant relationship to a positive DeMeester score. Because of the complexity of the procedure, expense, and general discomfort to the patient, it was hoped that detection of a defective LES would obviate the need for subsequent pHmonitoring in certain cases.
Manometric studies were performed using a 9-lumen catheter (ESM38R, Arndorfer, Greendale, Wisconsin) coupled to a hydraulic capillary infusion system (Arndorfer). The catheter consisted of a central lumen of 1.8 mm internal diameter surrounded by 8 lumens of 0.8 mm internal diameter. Four channels extended to the distal end of the catheter with ports at the same level and oriented radially at 90° intervals. The remaining 4 ports were spaced contiguously at 5 cm intervals proximal to, and offset by 45° from, the radial ports, providing 20 cm of working length. The central lumen was not used. The catheter was pulled at a 3 mm/second using a mechanical puller. The transducer information was translated into digital information using a polygraph (Medtronic Synectics, Shoreview, Minnesota). External transducers were also used to detect swallow and respiratory waves. The information was saved for real-time and retrospective review using Polygram software (Medtronic Synectics).
The original audit population consisted of 1900 patients referred to the Esophageal Function Laboratory in the Department of Surgery at Creighton University. This population received either esophageal manometry, 24-hour pHmonitoring or both. Patients were referred to the laboratory for typical or atypical symptoms thought to represent GERD. Of the 1900 patients in the referral database, only 627 received both esophageal manometry with measurement of LES vector volumes and ambulatory 24-hour pHmonitoring. Vector volume data were available for the total length of the lower esophageal sphincter (total vector volume, TVV) and for the intrabdominal length (intrabdominal vector volume, IVV). The vector volume is a calculated value representing the directional pressures within the LES over a specific portion of the sphincter. Additional data included age, weight, resting pressure of the LES (LESP), overall LES length (OL) and abdominal LES length (AL).
In preparation for station pull-through (SPT), the catheter assembly was introduced through either nare and advanced into the stomach. SPT was performed using a mechanized puller (Medtronic Synectics) at 3 mm/sec to avoid swallowing with pauses every centimeter to allow pressure measurement. Using the gastric baseline pressure as the reference point, a port entered the LES when a sustained positive deflection away from the baseline was observed. A wet swallow of 5 mL tap water was performed at this level to observe relaxation of the LES. The SPT proceeded through the entirety of the LES resulting in five measurements from separate channels which were averaged to produce the final value. The patient was instructed to breathe regularly and not to swallow. Swallowing (except for the wet swallow) required another attempt.
The resting pressure of the LES (LESP) was defined as the mean pressure at the respiratory inversion point (RIP). The RIP was the place on the pressure waveform where the positive deflections in the abdomen caused by inspiration changed to negative deflections in the chest. OL was the distance between the distal and proximal borders of the LES; AL was the distance between the distal border of the LES and the RIP. Both measurements were obtained during SPT.
Measurements for vector volume calculations were obtained from transducers attached to the 4 radial ports located at the same level on the catheter. Each channel yielded a separate pressure and direction (pressure vector) over the length of the LES. The distal border of the LES was defined as the position at which two or more of the four channels deflected positively from the gastric baseline. The crural component, often represented as the distal hump in the bimodal waveform, was intentionally included in the TVV. The proximal border was defined as that position in which two or more of the four channels returned and stayed below the gastric baseline. Computer software summated the pressure vector measurements into a single vector volume (mm Hg3). A three-dimensional image was also generated but not included in the database.
IVV was determined similarly. The proximal endpoint of the IVV was the RIP; the distal endpoint was the same as for TVV. As with TVV determination, when two or more of the four channels reached the RIP, all were considered to be at the RIP. Computer software similarly produced a value for the vector volume.
Upon completion of manometry, the pressure catheter was replaced with a pHprobe catheter (Ingold Bipolar Glass pHProbe, Mui Scientific, Mississauga, Ontario). All patients had been off their medication (PPI’s for 7 days, H2 blockers for 3 days) for the appropriate time. The catheter was passed through the nose and into the stomach to obtain a baseline reading from the ambulatory recording equipment to be carried by the patient (Digitrapper, Medtronic Synectics). The catheter was then withdrawn to a position 5 cm above the LES as determined by SPT. A baseline reading was again obtained. The catheter was secured to the nose and patients were given instructions to eat at least three meals (limited to foods in which the pHwas known), to stay upright at least 4 hours after eating, and to be recumbent for no more than 8 hours during sleep. Patients were instructed to note in a diary the time of each symptom occurrence, when and what they ate, and when they retired for bed.
At the completion of the 24-hour period, the catheter was removed and the information in the Digitrapper was downloaded and analyzed using pH-metry software (EsopHagram, Synectics). A DeMeester score was then tabulated by the computer and recorded for both acid and alkaline reflux. A score < 14.8 was considered negative for GERD.
Except for the logistic regression, sensitivities and specificities for the receiver-operating characteristics (ROC) were calculated manually. The purpose of the ROC was to find an optimal cut-off point, if any, for measurements that would yield the greatest combination of sensitivity and specificity. The formulas for calculations involving LESP (for example) were:
Sensitivity = [‘n’ patients with GERD and LESP ≤‘x’] ÷ [‘n’ patients with GERD]
Specificity = [‘n’ patient without GERD and LESP > ‘x’] ÷ [‘n’ patient without GERD]
In those calculations, ‘x’ refers to a progressively larger cut-off value for LESP (following the example above). Therefore, a particular sensitivity represented the probability that a patient with an LESP ≤‘x’ would have GERD. Likewise, the specificity represents that probability that a patient with an LESP > ‘x’ would not have GERD. Cut-off values were chosen at increments that resulted in enough data points to provide adequate resolution to the curve but with enough cases within each range to reflect real trends. This was done for LESP, TVV, IVV, AL and OL.
All other calculations were performed by SAS statistical software (version 6.12). The logistic regression process was automated and resulted in a formula for predicting GERD-positive or GERD-negative. According to its program, the statistical software started with all predictors in a test formula, then through backward selection, removed predictors that failed to meet the appropriate confidence interval. The software rejected predictors when p-values were > 0.05%. There were no attempts at relating the manometry measurements directly to DeMeester score in a continuous fashion. Rescaling in order to account for outliers consisted of setting values above the 95th percentile for any measurement equal to the value of that measurement at the 95th percentile. Values for normal were derived from a prior study in this laboratory using 50 healthy (non-GERD) volunteers. No attempts were made to sort patients or data according to age, sex or weight.
Demographic data with respect to the diagnosis of GERD is presented in Table 1. There were no attempts at age or sex matching. Table 2 includes the means, standard deviations, p-values, sensitivities and specificities for the measured parameters. The sensitivity and specificity of LESP, TVV and IVV, respectively, were 67.3% and 73.3%; 72.5% and 62.6%; and 58.5% and 77.3% at the lower values for normal. The sensitivity and specificity for AL and OL reflect the fact that very few patients had abnormal values of either (4 and 2 patients, respectively); this was also reflected in the similarity relatively large standard deviations between the average AL and OL for GERD and non-GERD suffers. Because AL and OL were so weakly sensitive for reflux disease, Any-low refers only to cases where LESP, TVV or IVV were below normal. The sensitivity of Any-low indicated an 81.4% probability a patient with either abnormally low LESP, TVV or IVV will have GERD. Similarly, AL and OL were also excluded from calculations of the All-low values. There was an 86.9% probability that a patient with all three normal measurements will not have an abnormal esophageal acid exposure; this is the specificity for All-low.
| Range | non-GERD mean ± SD | GERD mean ± SD | |
| Age (years) | 4-86 | 47.0 ± 16.1 (n = 308) | 48.5 ± 14.9 (n = 319) |
| Weight (lbs) | 87-397 | 173.6 ± 42.2 (n = 310) | 190.2 ± 38.0 (n = 317) |
| Sex | F: 313 M: 314 | F: 101 M: 80 | F: 108 M: 162 |
| Normal range | Range of measurements | non-GERD mean±SD | GERD mean±SD | P | Sens.% | Spec.% | |
| LESP | 6.1-55.6 | 0-59.3 | 10.9 ± 8.4 (n = 319) | 5.7 ± 4.8 (n = 308) | < 0.001 | 67.3 | 71.3 |
| IVV | 1855-10953 | 0-45830 | 4090 ± 4709 (n = 321) | 1712 ± 080 (n = 306) | < 0.001 | 72.5 | 62.6 |
| TVV | 2060-14135 | 80-60740 | 5429 ± 5564 (n = 321) | 2533 ± 2626(n = 306) | < 0.001 | 58.5 | 77.3 |
| AL | 1.0-5.0 | 0-9.8 | 2.6 ± 1.0 (n = 280) | 2.2 ± 1.0 (n = 347) | 0.013 | 4.5 | 98.4 |
| OL | 2.4-5.5 | 1.2-27.4 | 4.7 ± 1.8 (n = 287) | 4.6 ± 1.4 (n = 340) | < 0.047 | 2.6 | 99 |
| Any low | - | - | - | - | - | 81.4 | 51 |
| Allow | - | - | - | - | - | 49.3 | 86.9 |
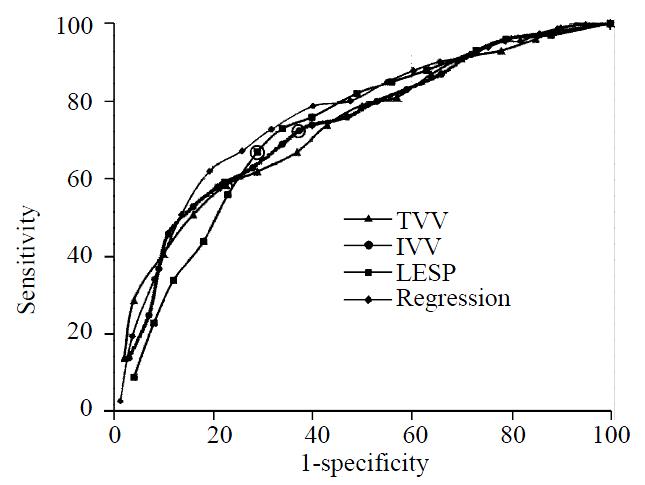
Figure 1 shows the receiver-operating characteristics for LESP, TVV, and IVV. The curves for AL and OL fell below all of the other curves and (for clarity) have not been depicted. There are no obvious points at which both specificity and sensitivity are optimal for any of the measurements. Cut-off values equal to the lowest normal values are indicated by the circled data points. Also shown in Figure 1 is an ROC curve for the regression formula (below) indicating that it does not have a better combination of sensitivity and specificity than the measurements themselves.
The logistic regression formula following backward selection was
Y = 1.24 + [LESP] (-0.1056) + [IVV] (-0.00019)
where Y is equal to a nominal value for GERD (1 = the presence and 2 = absence of GERD). P-values for the factor estimates were all < 0.0001. Various attempts were made at rescaling the data (as described in Materials and Methods) and ranking without effect. The “native” logistic regression (unscaled and unranked) was superior or equal in every case with 76.1% concordance, 23.6% discordance and 0.3% tied. Table 3 contains data from the logistic regression process. TVV, OL and AL were excluded by the software from the regression formula because of P-values > 0.05%. When ranked values were used for logistic regression, similar estimates were obtained for the y-intercept, LESP and IVV factors and the P-values were all < 0.0001. The formulas for the ranked and rescaled data were equally good or worse at predicting GERD and have been excluded from further discussion.
| Variable | Estimate | P |
| Intercept | +1.241 | < 0.0001 |
| LESP | -0.106 | < 0.0001 |
| IVV | -0.00019 | < 0.0001 |
| TVV | (rejected) | 0.43 |
| AL | (rejected) | 0.089 |
| OL | (rejected) | 0.79 |
| Concordant = 76.1% | ||
| Discordant = 23.6% | ||
| Tied = 0.3% |
Intraabdominal vector volume is more sensitive than TVV or LESP, but only marginally, and no more than 72.5% (Table 2). Measurements of TVV and LESP are more specific for GERD; that is, findings of normal TVV and LESP are 77.3% and 71.3% likely in a non-GERD patient, respectively. While the mean values for LESP, TVV and IVV are all significantly lower in GERD patients (P < 0.0001), the standard deviations are nearly equal to the means themselves in every measurement (Table 2). Intrabdominal length (AL) and overall length (OL) of the LES are clearly unimportant in the assessment of gastroesophageal reflux disease. Only 4 and 2 patients, respectively, had abnormally low values for AL or OL.
When measurements are viewed as ROC curves (Figure 1), there are no points where a measurement has both a high sensitivity and high specificity. That is to say that there appears to be no optimal cut-off value for predicting GERD. Ideally, an ROC curve is a parabolic figure with the apex in the upper right corner (using the axes as in Figure 1) which drops sharply from a point where sensitivity and specificity are both high to a point where sensitivity is very low and specificity is very high. In fact, there are no values for which there is a high sensitivity and even a moderately useful specificity. At cut-off values representing 85% sensitivity for GERD (≤ 1600 mm-Hg3 for TVV; ≤ 1000 mm-Hg3 for IVV; and ≤ 3 mm-Hg for LESP), the specificities of the measurements fall between 35% and 45%. Finding significantly lower-than-average TVV, IVV and LESP in GERD patients (Table 2) reiterates our understanding of the pathological consequences of a weakened LES. It also confirms our present understanding that there is a more complex relationship between the anatomical correlates of the LES and function than can be expressed by a single measurement.
Use of the “Any-low,”“All-low,” and logistic regression models were attempts to use the measurements in a combined fashioned, similar to the calculation of the DeMeester ambulatory pHscore. While Any-low had a relatively better sensitivity (81.4%) and All-low had relatively better specificity (86.9%) for the DeMeester score than the original measurements, the logistic regression-presumably the more rigorous mathematical model-was little better than the ROC curves for LESP, IVV or TVV. The computer generated a series of sensitivities and specificities for predictions made by the regression formula. This data is represented in Figure 1 as a means of comparison. Interestingly, total vector volume of the LES (TVV) was rejected by the computer due to a failure to meet the 95% correlation confidence interval. The logistic regression formula was only 76.1% accurate in predicting the real data. Attempts at ranking the data and rescaling in order to account for a number of outlying measurements made no improvement in the accuracy of the formula. The regression formula was clearly unable to improve on the predictive power of the raw measurements. As such, the formula has no practical use in the laboratory.
We have been motivated to find a way to identify surgical candidates without directly measuring distal esophageal reflux because of the inherent difficulty with ambulatory pHmonitoring (patient discomfort, cost, compliance). Recently, Fass and colleagues have suggested that the test itself may have the effect of reducing reflux-provoking activities, thereby reducing the sensitivity of the test[16]. In studies of different patient populations where vector volumes were found to have greater use[11-14] we are unaware of the degree to which they were able to control patient compliance with ambulatory pHmonitoring protocols (either maintaining a normal daily diet and activities or maintaining a controlled regimen). Certainly, patient compliance would be expected to be higher when he or she is made to understand the importance of maintaining normal habits. This is also an uncontrolled factor in the present study. In a sense, this might be a form of “compliance bias” reflected in the sensitivity of the test, where poor compliance (relatively less daily activity, smaller meals, more conservative food choices) results in more falsely negative screening tests. Quigley has also referred to the multiple difficulties with pH-metry as the gold standard for diagnosing GERD, namely the issue of patient compliance with testing conditions[17]. It remains to be seen if patient compliance, or any other factors, have any real effect on 24-hour pHmonitoring and the treatment indications for GERD.
To improve surgical results, it is helpful but not mandatory to find evidence that a defective lower esophageal sphincter is the cause for GERD. This is based on the reports by numerous authors who have sought to describe the sphincter and its mechanisms of failure[1-10]. Studies have demonstrated the effectiveness of anti-reflux surgery to improve symptom scores, esophagitis and LES resting pressure[2,7,11,13]. Authors have asserted that measurement of vector volumes would be superior to standard lower esophageal manometry for detecting a defective LES[11-15]. Wetscher and colleagues made the observation that IVV was more sensitive than LESP (i.e. standard manometry) and TVV at detecting a defective lower esophageal sphincter[12]. They concluded that measurement of vector volumes would be a valuable adjunct to current esophagogastric studies. Our study suggests that measurement of vector volumes is not greatly more valuable than traditional manometry.
There is an 81.4% chance that a patient with either a low LESP, TVV or IVV (when all three are measured) will have a positive DeMeester score. Whether or not this would suffice as an indication for surgery would require inclusion of surgical outcome data into the analysis. Alternatively, a priority ranking system used by Martinez-Serna and colleagues might serve as an effective adjunct to manometry data[18]. Those authors found a positive association between high symptom priority ranking for heartburn and regurgitation and abnormal pHand manometry results. Analysis of traditional manometry, vector volumes, symptom scores and surgical outcomes will be necessary to confidently define indications for surgery without ambulatory pHmonitoring.
Transient relaxation of the lower esophageal sphincter may be an important etiology for pathologic reflux[8-19]. Dodds and colleagues found that 65% of reflux episodes in GERD patients were due to transient relaxation of the LES[8]. This finding is difficult to interpret when 82% of normal reflux in non-GERD patients can also be attributed to transient LES relaxation[20]. Sloan and colleagues found that abrupt increases in intrabdominal pressure resulted in a higher occurrence of reflux in GERD patients who also had hiatal hernia and low LES resting tone[21]. Kahrilas et al found the degree of separation of the LES from the crura is associated with the transient relaxations[22]. This separation can be detected by manometry and is described as the double hump.
Based on our findings, we draw the following conclusions: (1) a patient with a low TVV, IVV or LESP (when all three are measured) is 81.4% likely to have a positive DeMeester score; and (2) when LESP, TVV and IVV are normal, a patient is 86.9% likely to have a negative DeMeester score. This is a marginal improvement over the sensitivities and specificities of the native measurements, but the probabilities are not large enough to justify omission of the ambulatory pHstudy despite its inherent challenge to patient compliance and reliability. It appears that vectors volumes, particularly intrabdominal vector volume, are just as sensitive and specific for GERD as LES resting pressure, and thus cannot be considered superior in the evaluation of GERD.
Edited by Zhang JZ
| 1. | Liebermann-Meffert D, Allgöwer M, Schmid P, Blum AL. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31-38. [PubMed] |
| 2. | Stein HJ, Crookes PF, DeMeester TR. Three-dimensional manometric imaging of the lower esophageal sphincter. Surg Annu. 1995;27:199-214. [PubMed] |
| 3. | Bonavina L, Evander A, DeMeester TR, Walther B, Cheng SC, Palazzo L, Concannon JL. Length of the distal esophageal sphincter and competency of the cardia. Am J Surg. 1986;151:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 81] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Stein HJ, Liebermann-Meffert D, DeMeester TR, Siewert JR. Three-dimensional pressure image and muscular structure of the human lower esophageal sphincter. Surgery. 1995;117:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | DeMeester TR, Wernly JA, Bryant GH, Little AG, Skinner DB. Clinical and in vitro analysis of determinants of gastroesophageal competence. A study of the principles of antireflux surgery. Am J Surg. 1979;137:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 123] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Stein HJ, Barlow AP, DeMeester TR, Hinder RA. Complications of gastroesophageal reflux disease. Role of the lower esophageal sphincter, esophageal acid and acid/alkaline exposure, and duodenogastric reflux. Ann Surg. 1992;216:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 165] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Costantini M, Zaninotto G, Anselmino M, Boccù C, Nicoletti L, Ancona E. The role of a defective lower esophageal sphincter in the clinical outcome of treatment for gastroesophageal reflux disease. Arch Surg. 1996;131:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Dodds WJ, Dent J, Hogan WJ, Helm JF, Hauser R, Patel GK, Egide MS. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982;307:1547-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 648] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 9. | Crookes PF, Kaul BK, DeMeester TR, Stein HJ, Oka M. Manometry of individual segments of the distal esophageal sphincter. Its relation to functional incompetence. Arch Surg. 1993;128:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Byrne PJ, Stuart RC, Lawlor P, Walsh TN, Hennessy TP. A new technique for measuring lower oesophageal sphincter competence in patients. Ir J Med Sci. 1993;162:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Bombeck CT, Vaz O, DeSalvo J, Donahue PE, Nyhus LM. Computerized axial manometry of the esophagus. A new method for the assessment of antireflux operations. Ann Surg. 1987;206:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Wetscher GJ, Hinder RA, Perdikis G, Wieschemeier T, Stalzer R. Three-dimensional imaging of the lower esophageal sphincter in healthy subjects and gastroesophageal reflux. Dig Dis Sci. 1996;41:2377-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Stein HJ, DeMeester TR, Naspetti R, Jamieson J, Perry RE. Three-dimensional imaging of the lower esophageal sphincter in gastroesophageal reflux disease. Ann Surg. 1991;214:374-383; discussion 383-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | DeMeester TR, Wang CI, Wernly JA, Pellegrini CA, Little AG, Klementschitsch P, Bermudez G, Johnson LF, Skinner DB. Technique, indications, and clinical use of 24 hour esophageal pH monitoring. J Thorac Cardiovasc Surg. 1980;79:656-670. [PubMed] |
| 15. | Stein HJ, Korn O, Liebermann-Meffert D. Manometric vector volume analysis to assess lower esophageal sphincter function. Ann Chir Gynaecol. 1995;84:151-158. [PubMed] |
| 16. | Fass R, Hell R, Sampliner RE, Pulliam G, Graver E, Hartz V, Johnson C, Jaffe P. Effect of ambulatory 24-hour esophageal pH monitoring on reflux-provoking activities. Dig Dis Sci. 1999;44:2263-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Quigley EM. 24-h pH monitoring for gastroesophageal reflux disease: already standard but not yet gold? Am J Gastroenterol. 1992;87:1071-1075. [PubMed] |
| 18. | Martinez-Serna T, Tercero F, Filipi CJ, Dickason TJ, Watson P, Mittal SK, Tasset MR. Symptom priority ranking in the care of gastroesophageal reflux: a review of 1,850 cases. Dig Dis. 1999;17:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Holloway RH, Kocyan P, Dent J. Provocation of transient lower esophageal sphincter relaxations by meals in patients with symptomatic gastroesophageal reflux. Dig Dis Sci. 1991;36:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 142] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Schoeman MN, Tippett MD, Akkermans LM, Dent J, Holloway RH. Mechanisms of gastroesophageal reflux in ambulant healthy human subjects. Gastroenterology. 1995;108:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 199] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Sloan S, Rademaker AW, Kahrilas PJ. Determinants of gastroesophageal junction incompetence: hiatal hernia, lower esophageal sphincter, or both? Ann Intern Med. 1992;117:977-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 170] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Kahrilas PJ, Shi G, Manka M, Joehl RJ. Increased frequency of transient lower esophageal sphincter relaxation induced by gastric distention in reflux patients with hiatal hernia. Gastroenterology. 2000;118:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 207] [Article Influence: 8.3] [Reference Citation Analysis (0)] |