Published online Jan 15, 2003. doi: 10.3748/wjg.v9.i1.141
Revised: August 3, 2002
Accepted: September 12, 2002
Published online: January 15, 2003
AIM: To investigate functional change of dendritic cells (DCs) derived from allogeneic partial liver graft undergoing acute rejection in rats.
METHODS: Allogeneic (SD rat to LEW rat) whole and 50% partial liver transplantation were performed. DCs from liver grafts 0 h and 4 d after transplantation were isolated and propagated in the presence of GM-CSF in vitro. Morphological characteristics of DCs propagated for 4 d and 10 d were observed by electron microscopy. Phenotypical features of DCs propagated for 10 d were analyzed by flow cytometry. Expression of IL-12 protein and IL-12 receptor mRNA in DCs propagated for 10 d was also measured by Western blotting and semiquantitative RT-PCR, respectively. Histological grading of rejection were determined.
RESULTS: Allogeneic whole liver grafts showed no features of rejection at day 4 after transplantation. In contrast, allogeneic partial liver grafts demonstrated moderate to severe rejection at day 4 after transplantation. DCs derived from allogeneic partial liver graft 4 d after transplantation exhibited typical morphological characteristics of DC after 4 d’ culture in the presence of GM-CSF. DCs from allogeneic whole liver graft 0 h and 4 d after transplantation did not exhibit typical morphological characteristics of DC until after 10 d’ culture in the presence of GM-CSF. After 10 d’ propagation in vitro, DCs derived from allogeneic whole liver graft exhibited features of immature DC, with absence of CD40, CD80 and CD86 surface expression, and low levels of IL-12 proteins (IL-12 p35 and IL-12 p40) and IL-12 receptor (IL-12Rβ1 and IL-12Rβ2) mRNA, whereas DCs from allogeneic partial liver graft 4 d after transplantation displayed features of mature DC, with high levels of CD40, CD80 and CD86 surface expression, and as a consequence, higher expression of IL-12 proteins (IL-12 p35 and IL-12 p40) and IL-12 receptors (IL-12Rβ1 and IL-12Rβ2) mRNA than those of DCs both from partial liver graft 0 h and whole liver graft 4 d after transplantation (P < 0.001) was observed.
CONCLUSION: DCs derived from allogeneic partial liver graft undergoing acute rejection display features of mature DC.
- Citation: Xu MQ, Yao ZX. Functional changes of dendritic cells derived from allogeneic partial liver graft undergoing acute rejection in rats. World J Gastroenterol 2003; 9(1): 141-147
- URL: https://www.wjgnet.com/1007-9327/full/v9/i1/141.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i1.141
The shortage of donor organs remains a major obstacle to the widespread application of liver transplantation in patients with end-stage liver disease. Although split grafting was used to increase the number of donor livers, the accelerated rejection induced by liver regeneration[1,2] can interfere with the outcome of these liver grafts. Therefore, It is important to investigate the mechanism responsible for the accelerated rejection of split liver grafting.
After organ transplantation, interstitial donor dendritic cells (DCs) migrate to recipient lymphoid tissue. In the case of experimental skin, heart, or kidney allografts, these cells have been implicated as the principal instigators of rejection. Despite similar patterns of donor DCs migration, liver grafts are accepted without immunosuppressive therapy between MHC-mismatched mouse, and certain rat strains, and induce donor-specific tolerance. These phenomena and the persistence of donor hematopoietic cells, including DCs, in successful, long-term graft recipients, have raised important questions about the possible role of donor DCs in liver transplant tolerance. The capacity of DCs to initiate immune responses is determined by their surface expression of MHC gene products and costimulatory molecules (CD40, CD80 and CD86), and the secretion of the immune regulator, interleukin (IL)-12[3-12]. Immature DCs resident in nonlymphoid tissues such as normal liver are deficient at antigen capture and progressing[3,13], whereas mature DCs, resident in secondary lymphoid tissues, are potent antigen-presenting cells, which can induce naive T-cell activation and proliferation[3-7]. Immature DCs that express surface MHC class II, but that are deficient in surface costimulatory molecules, can induce T-cell hyporesponsiveness[13-15] and inhibit immune reactivity[16,17]. It has been observed that liver-derived[13,18] or bone marrow-derived immature DCs[17], propagated in vitro and lacking surface costimulatory molecules, can prolong heart or pancreatic islet allograft survival. Whereas, marked augmentation of DCs numbers and maturation of DCs in liver allografts by donor treatment with the hematopoietic growth factor fms-like tyrosine kinase 3 (Flt3) ligand (FL) results in acute liver graft rejection[19,20].
Recent findings have revealed increased immune responses to regenerating allogeneic partial liver graft in rats[1,2], but little is known about the mechanism responsible for the accelerated rejection. The purpose of this study was to investigate the property of DCs isolated from allogeneic partial liver graft in comparison with DCs isolated from allogeneic whole liver graft, in an attempt to elucidate the possible mechanism responsible for the accelerated rejection of allogeneic partial liver graft. Given mature DC can induce naive T-cell activation and proliferation[3-7], and consequently induce acute rejection[19,20], we suspected that maturation of DCs derived from liver graft would be a key inducer of the accelerated rejection of allogeneic partial liver graft. In the present study, we first demonstrated that DCs derived from partial liver graft undergoing acute rejection displayed features of mature DC, including positive expression of costimulatory molecules, higher level expression of IL-12 protein and IL-12 receptor mRNA in these mature DCs.
One hundred male LEW rats and one hundred male SD rats weighing 220-300 g were used in all the experiments. Allogeneic whole and 50% partial liver transplantation were performed using a combination of SD rats to LEW rats. The animals were purchased from Chinese Academy of Science and Sichuan University. They were maintained with a 12-h light/dark cycle in a conventional animal facility with water and commercial chow provided ad libitum, with no fasting before the transplantation.
All operation were performed under ether anesthesia in clean but not sterile conditions. All surgical procedures were performed from 8 a.m. to 5 p.m. Donors and recipients of similar weight (± 10 g) were chosen. Liver reduction was achieved by removing the left lateral lobe and the two caudate lobes, which resulted in a 50% reduction of the liver mass. Whole liver transplantation (WLT) and partial liver transplantation (PLT) were performed with the two-cuff method described by Kammada and Calne[21], Knoop et al[22] and Uchiyama et al[23]. Briefly, the whole and partial liver were perfused with 20 ml of chilled lactated Ringer’s solution containing 200 U of heparin through the aorta. The liver was removed and immersed in chilled University of Wisconsin solution. Immediately after cuffs were placed on the portal vein and the infrahepatic vena cava, the liver graft was perfused with 8 ml of chilled University of Wisconsin solution through the portal vein and 2 ml of the same solution through the hepatic artery, and then the hepatic artery was ligated. Cold preservation time was approximately 1 h in all experiments. After a total hepatectomy was performed in the recipient, the suprahepatic vena cava was anastomosed in a continuous fashion with 8-0 sutures. The portal vein and infrahepatic vena cava were then connected by a 6-FG and 8-FG polyethylene tube cuff, respectively. The bile duct was internally stented with a 22-gauge i.v. catheter. The portal vein was clamped for < 18 min in all animals. In each group, the survival rate after grafting was > 89% at 24 h after surgery. Deaths that occurred within this period were defined as resulting from technical failure. Penicillin was given perioperatively.
Part of liver graft tissues 4 d after transplantation were sectioned and preserved in 10% Formalin, embedded in paraffin, cut with microtome, and stained with hematoxylin and eosin.
DCs from liver graft 0 h and 4 d after transplantation were propagated in GM-CSF from nonparenchymal cells (NPC) isolated from collagenase-digested liver graft tissue, as described by Lu[18]. Nonadherent cells, released spontaneously from proliferating cell clusters, were collected after 4 d’ and 10 d’ culture, and purified by centrifugation 500 × g, 10 min at room temperature on a 16% w/v metrizamide gradient (DC purity 80%-85%).
Morphological characteristics of DCs derived from liver graft were observed by electron microscopy. Expression of cell surface molecules was quantitated by flow cytometry as described by Mehling et al[4]. Aliquots of 2 × 105 DCs propagated for 10 d in vitro were incubated with the following primary mouse anti-rat mAbs against OX62, CD40, CD80, CD86 (Serotec, USA), or rat IgG as an isotype control for 60 min on ice (1 μg/ml diluted in PBS/1.0% FCS). The cells were washed with PBS/1.0% FCS and labeled with FITC-conjugated goat anti-mouse IgG, diluted 1/50 in PBS/1.0% FCS for 30 min on ice. At the end of this incubation cells were washed, PBS were added, and the cells were subsequently analyzed in an FACS-4200 flow cytometer (Becton-Dikison, USA).
Analysis of expression of IL-12Rβ1 and IL-12Rβ2 mRNA was determined by reverse transcription-PCR (RT-PCR) amplification in contrast with house-keeping gene GAPDH. Total RNA from 1 × 107 DCs propagated for 10 d in vitro was isolated using TripureTM reagent (Promega, USA). First-strand cDNA was transcribed from 1 μg RNA using AMV and an Oligo (dT15) primer. PCR was performed in a 25 μL reaction system containing 10 μL cDNA, 2 μL 10 mM dNTP, 2.5 μL 10 × buffer, 2.5 μL 25 mmol·L-1 MgCl2, 2 μL specific primer, 5 μL water and 1 μL Taq. IL-12Rβ1 and IL-12Rβ2 were amplified using specific primer for IL-12Rβ1[24] and IL-12Rβ2[25]. Specific primers for GAPDH[26] were also used for control. Thermal cycling of IL-12Rβ1 and IL-12Rβ2 and GAPDH primers were performed as follows[25]: denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, and extention at 72 °C for 1 min, all cycling were performed for 35 cycles. The predicted PCR product size were 331 bp for IL-12Rβ1, 1200 bp for IL-12Rβ2 and 576 bp for GAPDH. PCR products of each sample were subjected to electrophoresis in a 15 g·L-1 agarose gel containing 0.5 mg·L-1 ethidium bromide. Densitometrical analysis using NIH image software was performed for semiquantification of PCR products, and the expression level of each sample were expressed by IL-12R mRNA/GAPDH mRNA (%).
DCs cultured for 10 d in vitro were starved in serum-free medium for 4 h at 37 °C. These cells were washed twice in cold PBS, resuspended in 100 μL lysis buffer (1% Nonidet P-40, 20 mM Tris-HCl, pH8.0, 137 mM NaCl, 10% glycerol, 2 mM EDTA, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM PMSF, and 1 mM sodium orthovanadate), and total cell lysates were obtained. The homogenates were centrifuged at 10000 × g for 10 min at 4 °C. Cell lysates (20 μg) were electrophoresed on SDS-PAGE gels, and transferred to PVDC membranes for Western blot analysis. Briefly, PVDC membranes were incubated in a blocking buffer for 1 h at room temperature, then incubated for 2 h with Abs raised against IL-12 p35 (M-19, goat anti-rat, Santa Cruz, CA), IL-12p40 (H-306, rabbit anti-rat, Santa Cruz, CA). The membranes were washed and incubated for 1 h with HRP-labeled horse anti-goat or HRP-labeled goat anti-rabbit IgG. Immunoreactive bands were visualized by ECL detection reagent.
Statistic analysis of data was performed using the Student’s t-test; P < 0.05 was considered statistically significant.
Histology features of allografted livers were compared between the whole and partial groups on Day 4 after transplantation, allogeneic whole liver grafts demonstrated no rejection. In contrast, partial liver grafts demonstrated moderate to severe rejection, including inflammatory cellular infiltration in the portal tract, andothelialitis and bile duct damage.
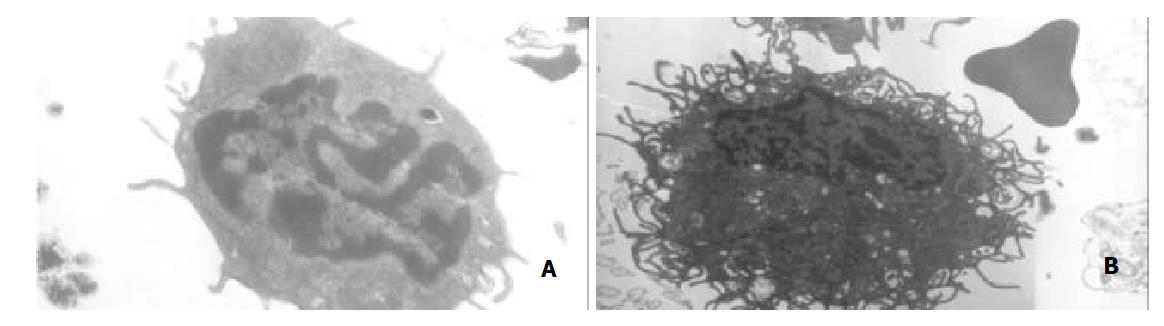
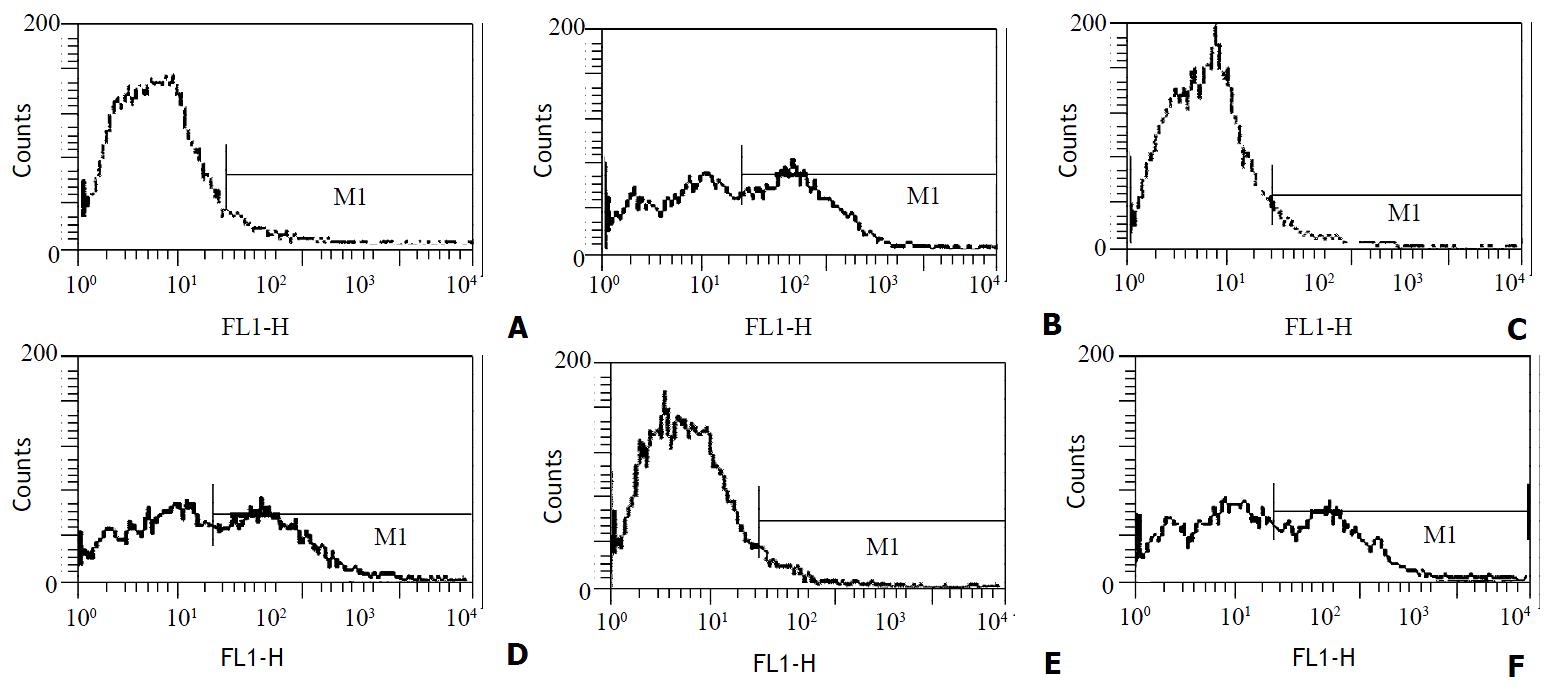
After 4 d’ culture in the presence of GM-CSF, liver graft-derived DCs were observed with electron microscopy, DCs from whole liver grafts 0 h and 4 d after transplantation exhibited round shape, smaller body, bigger nucleus, and a few shorter dendrites, whereas DCs derived from partial liver graft 4 d after transplantation displayed typical morphological features of DC including anomalous shape, bigger body, and numerous longer dendrites (as shown in Figure 1). Flow cytometry showed 80%-85% of DCs both from whole and partial liver grafts strongly expressed rat DC-specific OX62 antigen molecule (as shown in Table 1), which was suggested high purity DCs were obtained. After 10 day’ culture in the presence of GM-CSF, although DCs both from whole and partial liver grafts exhibited typical morphological features of DC by electron microscopy, flow cytometric analysis showed (as shown in Figure 2 and Table 1) whole liver graft-derived DCs displayed low amounts of costimulatory molecules (CD40, CD80 and CD86), whereas DCs from partial liver graft 4 d after transplantation expressed moderate to high levels of these markers. These results suggested allogeneic partial liver transplant could promote maturation of DCs derived from partial liver graft.
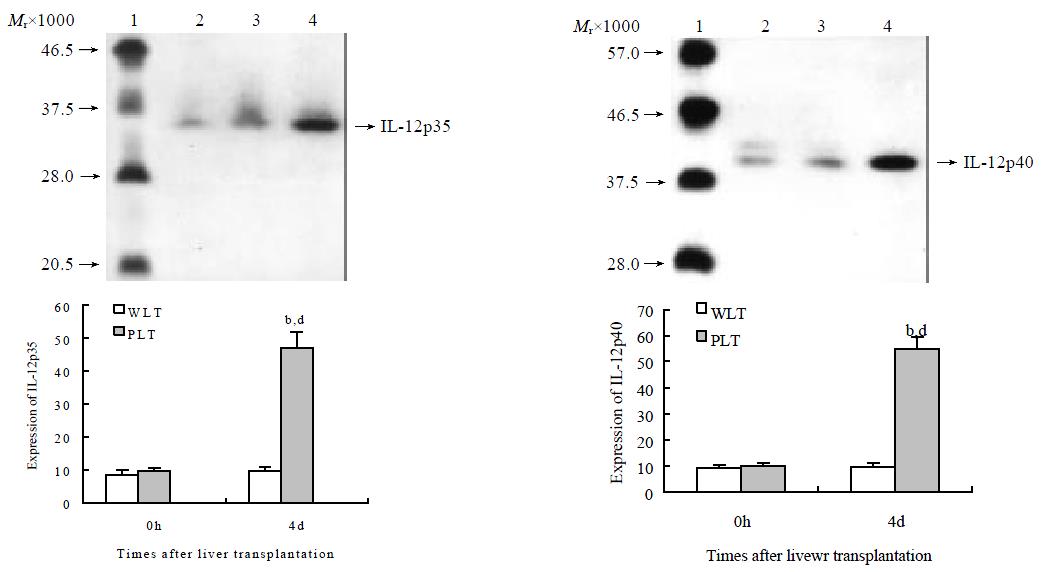
To investigate the functional change of DCs derived from liver grafts, we evaluated IL-12 protein expression in these DCs. As shown in Figure 3, DCs derived from both whole and liver grafts 0 h after transplantation expressed detectable but low levels of IL-12 p35 and IL-12 p40, and expression levels of IL-12 p35 and IL-12 p40 in DCs from whole liver graft 4 d after transplantation were similar to those of DCs from whole liver graft 0 h after transplantation (P > 0.05). However, expression of IL-12 p35 and IL-12 p40 in DCs from partial liver graft 4 d after transplantation was markedly increased, and their expression levels were significantly higher than those of DCs both from partial liver graft 0 h and whole liver graft 4 d after transplantation (P < 0.001).
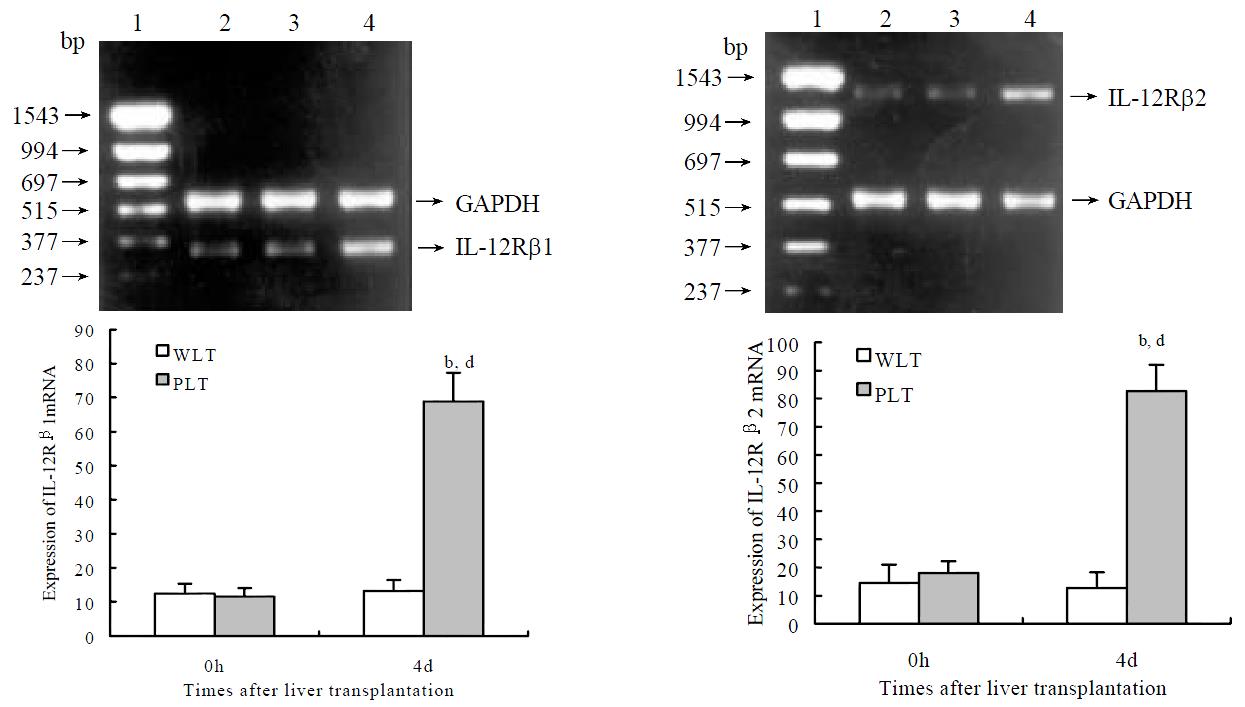
As shown in Figure 4, semiquantitative RT-PCR analysis revealed detectable but low levels of IL-12Rβ1 and IL-12Rβ2 mRNA expression in DCs both from whole and partial liver grafts 0 h after transplantation, expression levels of IL-12Rβ1 and IL-12Rβ2 mRNA in DCs from whole liver graft 4 d after transplantation were not markedly changed compared with those of DCs from whole liver graft 0 h after transplantation (P > 0.05). Whereas DCs from partial liver graft 4 d after transplantation expressed higher levels of IL-12Rβ1 mRNA and IL-12Rβ2 mRNA, and their expression levels were markedly higher than those of DCs both derived from partial liver graft 0 h and whole liver graft 4 d after transplantation (P < 0.001).
DCs play critical roles in the initiation and modulation of immune responses[27-34]. After vascularized organ transplantation, donor passenger leukocytes (mainly interstitial DCs) are mobilized out of the graft via peripheral blood to the recipient lymphoid and nonlymphoid tissues[35-39]. Maturation of donor DC from thyroid, pancreatic islet, skin, or kidney allografts in recipient lymphoid organs lead to the activation of naive, alloreactive Th0 lymphocytes, and thus provides the primary stimulus for acute allograft rejection. However, in mouse and certain rat strain combinations, fully MHC-mismatched liver allografts accepted without any of immune suppression, and fail to elicit an effective rejection response[38,39]. Moreover, in humans, the liver is considered the least immunogenic of transplanted whole organs. In a tolerant rat strain combination, depletion of interstitial leukocytes from liver by pretransplant donor radiation prevents the tolerogenic effect, and results in acute rejection[40]. On the other hand, it has been reported that immature, costimulatory molecule-deficient DCs (such as normal liver or bone marrow-derived DCs) propagated in vitro can promote graft survival in allogeneic recipients[14,41,42], and posttransplant administration of donor leukocytes induces long-term acceptance of liver transplants[43].
Therefore, passenger leukocytes (most likely DCs) may have a dualistic role with potential to elicit T cell activation and graft rejection, or induce T cell tolerance and graft acceptance. The sustained release from the transplanted liver of immature DCs, may contribute to allogeneic liver graft tolerance induction. These liver-derived DCs migrate in vivo to T cells areas of secondary lymphoid tissue, where they persisted for weeks in allogeneic recipients[18,44]. It is accepted that alloantigen-specific Th1 cells initiate allograft rejection, and that Th2 cells exert an inhibitory influence on the development of Th1 clones. It has been proposed that preferential induction of alloantigen-specific Th2 lymphocytes could suppress the development of Ag-specific Th1 cells, and as a consequence, inhibit allograft rejection. Liver-derived DCs might induce the proliferation of Th2 clones with capacity to inhibit Th1 responses[18]. Liver-derived DCs display an immature phenotype with absence of costimlatory molecules (CD40, CD80 and CD86) surface expression, low levels of MHC classIandII, and as a consequence, low stimulatory capacity for naive allogeneic T cells. Unlike mature DC, these liver-derived DCs do not induce detectable levels of intracytoplasmic IFN-γ in allogeneic CD4+ cells in 72-h MLR, and elicited very low levels of CTLs in vitro[3,13,18]. In contrast, acute liver graft rejection would be induced by maturation of liver grafts derived-DCs[20]. These findings point to a pivotal role for donor immature or mature DCs in determining the outcome of liver transplantation. Mature DCs express high levels of costimulatory molecules such as CD40, CD80 and CD86. Activation of T cells by mature DCs has been shown to require direct contact between T cells and DCs through CD40-CD40L interaction[45], upon ligation of CD40L on T cells with CD40 on DCs, DCs are triggered to produce even high quantities of IL-12, thus consigning T cells to Th1 responses[46].
IL-12 is an important immune regulator produced primarily by DCs and macrophages that drives the preferential induction of Th1 immune responses. IL-12 appears to be a central mediator of acute graft-vs-host disease in mice[47], whereas neutralization of bioactive IL-12 enhances allogeneic myoblast survival[48]. Moreover, exogenous IL-12 mediates liver allograft rejection[49], and IL-12 antagonism could promote liver graft tolerance[19]. IL-12 binds to a unique, high affinity receptor on activated Th cells and NK cells, enhances the expression of antiapoptotic factors (bcl2 and bclxl), and facilitates activated T cell and NK-lymphokine activated killer cell expansion. IL-12 indirectly promotes Th1 and inhibits Th2 development by inducing the secretion of IFN-γ by Th1 and NK cells[19,50-54]. Recent investigation showed IL-12 also induce autologus IL-12 production of DC by interaction with IL-12 receptor[25]. Previous studies have shown that IL-12R is detected in T cells and NK cells, and IL-12R plays a crucial role for IL-12 mediated activation of these cell types[25,55-60]. IL-12Rβ1 is the subunit primarily responsible for binding IL-12, and IL-12Rβ2 plays an essential role in mediating the biological functions of IL-12. IL-12-induced phosphorylation of STAT4 and IFN-γ production are absent in Con A and anti-CD3-activated splenocytes from IL-12Rβ2-/- mice[56]. Recent investigations suggested that DCs exhibited expression of IL-12Rβ1 and IL-12Rβ2[25,61-66], and mature DCs express high level of IL-12Rβ1 and IL-12Rβ2[25,64].
Although Omura T et al[1] and Shiraishi M et al[2] reported that allogeneic partial liver grafts exhibited increased immune response compared with allogeneic whole liver grafts as early as 3 d after transplantation, little is known about the exact mechanism responsible for the accelerated rejection. In the present study, accelerated rejection was demonstrated in allogeneic partial liver graft 4 d after transplantation. Our results first demonstrated that DCs derived from allogeneic whole liver graft without acute rejection 4 d after transplantation exhibited an immature phenotype with absence of CD40, CD80 and CD86 surface expression, and low expression of IL-12 proteins (IL-12 p35 and IL-12 p40) and IL-12 receptor (IL-12Rβ1 and IL-12Rβ2) mRNA. In contrast with immature DCs derived from whole liver graft, DCs derived from partial liver graft undergoing acute rejection 4 d after transplantation displayed a mature phenotype with high level of CD40, CD80 and CD86 surface expression, and as a consequence, high level expression of IL-12 proteins (IL-12 p35 and IL-12 p40) and IL-12 receptors (IL-12Rβ1 and IL-12Rβ2) mRNA. Given immature DCs can induce T-cell hyporesponsiveness[13-15] and immune reactivity inhibition[16,17], whereas mature DCs can stimulate Th1 response and the development of alloantigen-specific CTLs[3-7,27-34], together with IL-12 is an important inducer of liver graft rejection, we suggest that maturation of liver graft-derived DCs may be an important mechanism of the accelerated rejection of allogeneic partial liver graft, and inhibition of maturation of liver graft-derived DCs may suppress rejection of allogeneic partial liver graft.
Edited by Zhang JZ
| 1. | Omura T, Nakagawa T, Randall HB, Lin Z, Huey M, Ascher NL, Emond JC. Increased immune responses to regenerating partial liver grafts in the rat. J Surg Res. 1997;70:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Shiraishi M, Csete ME, Yasunaga C, Drazan KE, Jurim O, Cramer DV, Busuttil RW, Shaked A. Regeneration-induced accelerated rejection in reduced-size liver grafts. Transplantation. 1994;57:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Morelli AE, O'Connell PJ, Khanna A, Logar AJ, Lu L, Thomson AW. Preferential induction of Th1 responses by functionally mature hepatic (CD8alpha- and CD8alpha+) dendritic cells: association with conversion from liver transplant tolerance to acute rejection. Transplantation. 2000;69:2647-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Mehling A, Grabbe S, Voskort M, Schwarz T, Luger TA, Beissert S. Mycophenolate mofetil impairs the maturation and function of murine dendritic cells. J Immunol. 2000;165:2374-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Ismaili J, Rennesson J, Aksoy E, Vekemans J, Vincart B, Amraoui Z, Van Laethem F, Goldman M, Dubois PM. Monophosphoryl lipid A activates both human dendritic cells and T cells. J Immunol. 2002;168:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Sato K, Nagayama H, Enomoto M, Tadokoro K, Juji T, Takahashi TA. Autocrine activation-induced cell death of T cells by human peripheral blood monocyte-derived CD4+ dendritic cells. Cell Immunol. 2000;199:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Zheng H, Dai J, Stoilova D, Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J Immunol. 2001;167:6731-6735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167:2479-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Kobayashi M, Azuma E, Ido M, Hirayama M, Jiang Q, Iwamoto S, Kumamoto T, Yamamoto H, Sakurai M, Komada Y. A pivotal role of Rho GTPase in the regulation of morphology and function of dendritic cells. J Immunol. 2001;167:3585-3591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J Immunol. 2001;166:2444-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 256] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Thoma-Uszynski S, Kiertscher SM, Ochoa MT, Bouis DA, Norgard MV, Miyake K, Godowski PJ, Roth MD, Modlin RL. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol. 2000;165:3804-3810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Khanna A, Morelli AE, Zhong C, Takayama T, Lu L, Thomson AW. Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J Immunol. 2000;164:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Hirano A, Luke PP, Specht SM, Fraser MO, Takayama T, Lu L, Hoffman R, Thomson AW, Jordan ML. Graft hyporeactivity induced by immature donor-derived dendritic cells. Transpl Immunol. 2000;8:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Lee WC, Zhong C, Qian S, Wan Y, Gauldie J, Mi Z, Robbins PD, Thomson AW, Lu L. Phenotype, function, and in vivo migration and survival of allogeneic dendritic cell progenitors genetically engineered to express TGF-beta. Transplantation. 1998;66:1810-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Hayamizu K, Huie P, Sibley RK, Strober S. Monocyte-derived dendritic cell precursors facilitate tolerance to heart allografts after total lymphoid irradiation. Transplantation. 1998;66:1285-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Khanna A, Steptoe RJ, Antonysamy MA, Li W, Thomson AW. Donor bone marrow potentiates the effect of tacrolimus on nonvascularized heart allograft survival: association with microchimerism and growth of donor dendritic cell progenitors from recipient bone marrow. Transplantation. 1998;65:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Lu L, Woo J, Rao AS, Li Y, Watkins SC, Qian S, Starzl TE, Demetris AJ, Thomson AW. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med. 1994;179:1823-1834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 199] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Li W, Lu L, Wang Z, Wang L, Fung JJ, Thomson AW, Qian S. Il-12 antagonism enhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-treated donors and promotes graft acceptance. J Immunol. 2001;166:5619-5628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Steptoe RJ, Fu F, Li W, Drakes ML, Lu L, Demetris AJ, Qian S, McKenna HJ, Thomson AW. Augmentation of dendritic cells in murine organ donors by Flt3 ligand alters the balance between transplant tolerance and immunity. J Immunol. 1997;159:5483-5491. [PubMed] |
| 21. | Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64-69. [PubMed] |
| 22. | Knoop M, Bachmann S, Keck H, Steffen R, Neuhaus P. Experience with cuff rearterialization in 600 orthotopic liver grafts in the rat. Am J Surg. 1994;167:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Uchiyama H, Yanaga K, Nishizaki T, Soejima Y, Yoshizumi T, Sugimachi K. Effects of deletion variant of hepatocyte growth factor on reduced-size liver transplantation in rats. Transplantation. 1999;68:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Collison K, Saleh S, Parhar R, Meyer B, Kwaasi A, Al-Hussein K, Al-Sedairy S, Al-Mohanna F. Evidence for IL-12-activated Ca2+ and tyrosine signaling pathways in human neutrophils. J Immunol. 1998;161:3737-3745. [PubMed] |
| 25. | Nagayama H, Sato K, Kawasaki H, Enomoto M, Morimoto C, Tadokoro K, Juji T, Asano S, Takahashi TA. IL-12 responsiveness and expression of IL-12 receptor in human peripheral blood monocyte-derived dendritic cells. J Immunol. 2000;165:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Vos TA, Hooiveld GJ, Koning H, Childs S, Meijer DK, Moshage H, Jansen PL, Müller M. Up-regulation of the multidrug resistance genes, Mrp1 and Mdr1b, and down-regulation of the organic anion transporter, Mrp2, and the bile salt transporter, Spgp, in endotoxemic rat liver. Hepatology. 1998;28:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 249] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Zhang JK, Chen HB, Sun JL, Zhou YQ. Effect of dendritic cells on LPAK cells induced at different times in killing hepatoma cells.. Shijie Huaren Xiaohua Zazhi. 1999;7:673-675. |
| 28. | Li MS, Yuan AL, Zhang WD, Chen XQ, Tian XH, Piao YJ. Immune response induced by dendritic cells induce apoptosis and inhibit proliferation of tumor cells.. Shijie Huaren Xiaohua Zazhi. 2000;8:56-58. |
| 29. | Luo ZB, Luo YH, Lu R, Jin HY, Zhang PB, Xu CP. Immunohistochemical study on dendritic cells in gastric mucosa of patients with gastric cancer and precancerous lesions.. Shijie Huaren Xiaohua Zazhi. 2000;8:400-402. |
| 30. | Li MS, Yuan AL, Zhang WD, Liu SD, Lu AM, Zhou DY. Dendritic cells in vitro induce efficient and special anti-tumor immune response.. Shijie Huaren Xiaohua Zazhi. 1999;7:161-163. |
| 31. | Wang FS, Xing LH, Liu MX, Zhu CL, Liu HG, Wang HF, Lei ZY. Dysfunction of peripheral blood dendritic cells from patients with chronic hepatitis B virus infection. World J Gastroenterol. 2001;7:537-541. [PubMed] |
| 32. | Zhang JK, Li J, Chen HB, Sun JL, Qu YJ, Lu JJ. Antitumor activities of human dendritic cells derived from peripheral and cord blood. World J Gastroenterol. 2002;8:87-90. [PubMed] |
| 33. | Tang ZH, Qiu WH, Wu GS, Yang XP, Zou SQ, Qiu FZ. The immunotherapeutic effect of dendritic cells vaccine modified with interleukin-18 gene and tumor cell lysate on mice with pancreatic carcinoma. World J Gastroenterol. 2002;8:908-912. [PubMed] |
| 34. | Zhang J, Zhang JK, Zhuo SH, Chen HB. Effect of a cancer vaccine prepared by fusions of hepatocarcinoma cells with dendritic cells. World J Gastroenterol. 2001;7:690-694. [PubMed] |
| 35. | Maestroni GJ. Dendritic cell migration controlled by alpha 1b-adrenergic receptors. J Immunol. 2000;165:6743-6747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 346] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 37. | Thomson AW, Lu L, Murase N, Demetris AJ, Rao AS, Starzl TE. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995;13:622-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 140] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Jonsson JR, Hogan PG, Thomas R, Steadman C, Clouston AD, Balderson GA, Lynch SV, Strong RW, Powell EE. Peripheral blood chimerism following human liver transplantation. Hepatology. 1997;25:1233-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Spriewald BM, Wassmuth R, Carl HD, Köckerling F, Reichstetter S, Kleeberger A, Klein M, Hohenberger MW, Kalden JR. Microchimerism after liver transplantation: prevalence and methodological aspects of detection. Transplantation. 1998;66:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Sun J, McCaughan GW, Gallagher ND, Sheil AG, Bishop GA. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation. 1995;60:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Rastellini C, Lu L, Ricordi C, Starzl TE, Rao AS, Thomson AW. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation. 1995;60:1366-1370. [PubMed] |
| 42. | Gao JX, Madrenas J, Zeng W, Cameron MJ, Zhang Z, Wang JJ, Zhong R, Grant D. CD40-deficient dendritic cells producing interleukin-10, but no t interleukin-12, induce T-cell hyporesponsiveness in vitro and prevent acute allograft rejection.. Immunolgy. 1999;98:159-170. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Yan Y, Shastry S, Richards C, Wang C, Bowen DG, Sharland AF, Painter DM, McCaughan GW, Bishop GA. Posttransplant administration of donor leukocytes induces long-term acceptance of kidney or liver transplants by an activation-associated immune mechanism. J Immunol. 2001;166:5258-5264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Thomson AW, Lu L, Subbotin VM, Li Y, Qian S, Rao AS, Fung JJ, Starzl TE. In vitro propagation and homing of liver-derived dendritic cell progenitors to lymphoid tissues of allogeneic recipients. Implications for the establishment and maintenance of donor cell chimerism following liver transplantation. Transplantation. 1995;59:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121-1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 498] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 46. | Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1539] [Cited by in RCA: 1549] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 47. | Williamson E, Garside P, Bradley JA, Mowat AM. IL-12 is a central mediator of acute graft-versus-host disease in mice. J Immunol. 1996;157:689-699. [PubMed] |
| 48. | Kato K, Shimozato O, Hoshi K, Wakimoto H, Hamada H, Yagita H, Okumura K. Local production of the p40 subunit of interleukin 12 suppresses T-helper 1-mediated immune responses and prevents allogeneic myoblast rejection. Proc Natl Acad Sci USA. 1996;93:9085-9089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Thai NL, Li Y, Fu F, Qian S, Demetris AJ, Duquesnoy RJ, Fung JJ. Interleukin-2 and interleukin-12 mediate distinct effector mechanisms of liver allograft rejection. Liver Transpl Surg. 1997;3:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 519] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 51. | Shibuya K, Robinson D, Zonin F, Hartley SB, Macatonia SE, Somoza C, Hunter CA, Murphy KM, O'Garra A. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J Immunol. 1998;160:1708-1716. [PubMed] |
| 52. | Sin JI, Kim JJ, Arnold RL, Shroff KE, McCallus D, Pachuk C, McElhiney SP, Wolf MW, Pompa-de Bruin SJ, Higgins TJ. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J Immunol. 1999;162:2912-2921. [PubMed] |
| 53. | Bhardwaj N, Seder RA, Reddy A, Feldman MV. IL-12 in conjunction with dendritic cells enhances antiviral CD8+ CTL responses in vitro. J Clin Invest. 1996;98:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J Immunol. 1999;163:2561-2567. [PubMed] |
| 55. | Kim J, Uyemura K, Van Dyke MK, Legaspi AJ, Rea TH, Shuai K, Modlin RL. A role for IL-12 receptor expression and signal transduction in host defense in leprosy. J Immunol. 2001;167:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Wu C, Wang X, Gadina M, O'Shea JJ, Presky DH, Magram J. IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J Immunol. 2000;165:6221-6228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Wang KS, Frank DA, Ritz J. Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood. 2000;95:3183-3190. [PubMed] |
| 58. | Chang JT, Shevach EM, Segal BM. Regulation of interleukin (IL)-12 receptor beta2 subunit expression by endogenous IL-12: a critical step in the differentiation of pathogenic autoreactive T cells. J Exp Med. 1999;189:969-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Kawashima T, Kawasaki H, Kitamura T, Nojima Y, Morimoto C. Interleukin-12 induces tyrosine phosphorylation of an 85-kDa protein associated with the interleukin-12 receptor beta 1 subunit. Cell Immunol. 1998;186:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Lawless VA, Zhang S, Ozes ON, Bruns HA, Oldham I, Hoey T, Grusby MJ, Kaplan MH. Stat4 regulates multiple components of IFN-gamma-inducing signaling pathways. J Immunol. 2000;165:6803-6808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M, Koyasu S. Interleukin 12-dependent interferon gamma production by CD8alpha+ lymphoid dendritic cells. J Exp Med. 1999;189:1981-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 267] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 62. | Fukao T, Matsuda S, Koyasu S. Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-gamma production by dendritic cells. J Immunol. 2000;164:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 63. | Fukao T, Frucht DM, Yap G, Gadina M, O'Shea JJ, Koyasu S. Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J Immunol. 2001;166:4446-4455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Fukao T, Koyasu S. Expression of functional IL-2 receptors on mature splenic dendritic cells. Eur J Immunol. 2000;30:1453-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 65. | Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998;9:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 228] [Article Influence: 8.4] [Reference Citation Analysis (0)] |