Published online Jan 15, 2003. doi: 10.3748/wjg.v9.i1.112
Revised: June 23, 2002
Accepted: July 26, 2002
Published online: January 15, 2003
AIM: To establish a mice model harboring hepatitis B virus x gene (adr subtype) for studying the function of hepatitis B virus X protein, a transactivator of viral and cellular promoter/enhancer elements.
METHODS: Expression vector pcDNA3-HBx, containing CMV promoter and hepatitis B virus x gene open reading fragment, was constructed by recombination DNA technique. Hela cells were cultured in DMEM and transfected with pcDNA3-HBx or control pcDNA3 plasmids using FuGENE6 Transfection Reagent. Expression of pcDNA3-HBx vectors in the transfected Hela cells was confirmed by Western blotting. After restriction endonuclease digestion, the coding elements were microinjected into male pronuclei of mice zygotes. The pups were evaluated by multiplex polymerase chain reaction (PCR) at genomic DNA level. The x gene transgenic mice founders were confirmed at protein level by Western blotting, immunohistochemistry and immunogold transmission electron microscopy.
RESULTS: Expression vector pcDNA3-HBx was constructed by recombination DNA technique and identified right by restriction endonuclease digestion and DNA direct sequencing. With Western blotting, hepatitis X protein was detected in Hela cells transfected with pcDNA3-HBx plasmids, suggesting pcDNA3-HBx plasmids could express in eukaryotic cells. Following microinjection of coding sequence of pcDNA3-HBx, the embryos were transferred to oviducts of psedopregnant females. Four pups were born and survived. Two of them were verified to have the HBx gene integrated in their genomic DNA by multiplex PCR assay, and named C57-TgN (HBx)S MMU1 and C57-TgN (HB x) SMMU3 respectively. They expressed 17KD X protein in liver tissue by Western blotting assay. With the immunohistochemistry, X protein was detected mainly in hepatocytes cytoplasm of transgenic mice, which was furthermore confirmed by immunogold transmission electon microscopy.
CONCLUSION: We have constructed the expression vector pcDNA3-HBx that can be used to study the function of HBx gene in eukaryotic cells in vitro. We also established HBx gene (adr subtype) transgenic mice named C57-TgN (HBx) SMMU harboring HBx gene in their genome and express X protein in hepatocytes, Which might be a valuable animal system for studying the roles of HBx gene in hepatitis B virus life cycle and development of hepatocellular carcinoma in vivo.
- Citation: Xiong J, Yao YC, Zi XY, Li JX, Wang XM, Ye XT, Zhao SM, Yan YB, Yu HY, Hu YP. Expression of hepatitis B virus X protein in transgenic mice. World J Gastroenterol 2003; 9(1): 112-116
- URL: https://www.wjgnet.com/1007-9327/full/v9/i1/112.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i1.112
Human hepatitis B virus (HBV) is the prototype for a family of viruses, referred to as Hepadnaviridae[1,2]. It has at least 4 subtypes, ayw, adr, ayr, and adw, among which adr is the most prevailing subtype in China. The complete genomic DNA of subtype adr has been cloned and demonstrated only 3.2 kbp in length, andwhich is different from the other 3 subtypes in DNA and protein sequence[3]. HBV genome has 4 open reading frames (ORFs), including envelope genes coding region (pre-s1, pre-s2 and s gene coding region), precore (pc) gene and core(c) gene coding region, polymerase (p) gene coding region, x gene coding region[4-6].
Chronic HBV infection is associated with a high incidence of liver disease, including hepatocellular carcinoma (HCC)[7-9]. Based on epidemiologic studies involving chronic HBV infection, it is estimated that the relative risk of developing HCC for HBV carriers may be 100- to 200-fold higher than that for non-carriers. It is proposed that the role of HBV played in HCC predisposition is modifying host gene regulation. Integration of viral DNA into the host genome can mediate host gene deregulation by a variety of mechanisms[10-15]. X protein may alter host gene expression leading to the development of HCC[16]. It has been demonstrated that X protein is a transactivator of a variety of viral and cellular promoter/enhancer elements and can mediate the activation of signal transduction pathways. Besides, it may affect DNA repair, cell cycle control, and apoptosis[17-22]. It is now clear that X-defective virus is unable to initiate infection in vivo. However, the physiological role of X protein during the course of an infection remains a major issue unresolved in hepadnavirus biology[23-27].
To explore the function of HBx gene in vivo, we generated transgenic mice harboring HBx gene from subtype adr by microinjection method, in which HBx gene could be expressed. This model might be valuable for the study of HBx biology and its associated biomedical issues in vivo.
Restriction Endonucleases and T4 DNA ligase were obtained from Promega Co. (USA). The mouse monoclonal antibody against X protein was purchased from DAKO (USA). Sheep anti mouse IgG-HRP was obtained from CALBIOCHEM (Germany). Gel extraction kit was purchase from QIAGEN. Hela cells were preserved in our laboratory. C57BL/6 mice were maintained in our Transgenic Animal Laboratory (SPF level).
Plasmids pBR322-HBV (containing two tandem copies of the HBV genome of adr subtype) and pcDNA3 (containing CMV promoter) were preserved in our laboratory. Expression plasmid pcDNA3.1 (containing CMV promoter) was generously provided by Dr. Yu Hong-Yu. An 0.894-Kilobase pair DNA fragment containing HBx gene was isolated by gel extraction from plasmid pBR322-HBV after Hind III and Bgl II restriction digest. The fragment was then subcloned into plasmid pcDNA3.1 that has been digested by Hind III and BamH I to yield intermediate plasmid pcDNA3.1-HBx, which was employed as a template for polymerase chain reaction (PCR) amplification of the HBx coding fragments. The primers (A: 5’-ACACA AGCTT CATAT GGCTG CTCGG G-3’, B: 5’-CATGA ATTCT AGATG ATTAG GCAGA GGTG-3’) were synthesized by Sangon Co. (Shanghai). Thirty five cycles of amplification were done in a total volume of 50 μl with an annealing temperature of 58 °C. PCR product and pcDNA3 were isolated after Hind III and Xba I digestion. After ligation, the plasmid of pcDNA3-HBx was confirmed by restriction endonucleases digestion and direct DNA sequencing.
Hela cells were cultured in DMEM (Gibco) supplemented with 10% FCS (Gibco) to confluence. Cells at 50% confluency were transfected with pcDNA3-HBx or contol pcDNA3 plasmids using FuGENE6 Transfection Reagent (Roche) with a total of 1 ug of DNA per 3.5-cm plate of cells. Selection in medium containing geneticin (G418; Gibco) at a concentration of 500 μg/mL was started 48 hours later. After 2 weeks selection, positive clones that were named Hela-HBx were isolated and further expanded.
Hela-HBx cells cultured in 10-cm dishes were rinsed with phosphate-buffered saline (pH7.4) three times and collected in a microcentrifig tube by trypsinzation. Cells were lysed with lysis buffer[18]. Supernatants were then diluted 5 times with phosphate-buffered saline (pH7.4) to assay the expression of the transfected pcDNA3-HBx vectors in Hela cells by Western blotting.
The pcDNA3-HBx plasmid was digested by Sal I and purified by gel extraction (Qiagen gel extraction kit). Purified coding fragment containing CMV promoter and HBx ORF were dissolved in TE buffer (10 mM Tris-HCl, 0.2 mM EDTA, pH7.5) at a final concentration of 2 ug/L (-4000 copies/pl) and microinjected into zygotes. Microinjection and embryo manipulation were performed according to standard protocols.
Genomic DNA was extracted from tail tissue of pups mice or normal mouse and dissolved in TE buffer. It was used for PCR assays to identify founders of transgenic mice with HBx gene. In order to set an internal control of the efficiency of PCR amplification, we developed a multiplex PCR, using two sets of primers to amplify the HBx gene and the autosomal IL3 gene in the same reaction tube[28,29]. PCR reaction was performed using 1 μl of dissolved DNA, 0.2 μm HBx gene specific primers (C: 5’-GGACGTCCTTTGTCTACGTCCCGTC-3’, D: 5’-CCTAATCTCCTCCCCCAACTCCTCC-3’, synthesized by Sangon Co./Shanghai), and 0.1 μm IL3 gene specific primers (E: 5’-GGGAC TCCAA GCTTC AATCA-3’, F: 5’-TGGAGGAGGAAGAAAAGCAA-3’, synthesized by Sangon Co./Shanghai) in a total volume of 50 μl according to the cycling program: 94 °C, 40 s; 61 °C, 40 s; 72 °C, 60 s; 35 cycles.
Western blotting Liver samples were obtained from the transgenic mice with HBx gene and normal C57BL/6 mice. Specimens (approximately 100 mg) were homogenized in a screw-capped 1.5 mL microcentrifage tube and lyzed in lysis buffer (0.5% Nonidet P-40, 10 mM Tris (pH7.4), 150 mM NaCl, 1 mM EDTA and 1 mM phenylmethanesulfonyl fluorid). 100 mg lysate was separated via 15% SDS-polyacrylamide gel electrophoresis with Tris-Glycine buffer (pH8.3). One electrophoresis gel was stained with commassie brilliant blue R-250, and another was blotted to nitorcellulose filter. After blocked with 50 g/L defatted milk, the filter was incubated with X protein mouse monoclonal antibody for 40 min at 37 °C, then washed with TBS (three times, 15 min each time) and incubated with HRP-conjugated sheep anti-mouse IgG for 30 min at 37 °C. Finally, the filter was incubated with peroxidase substrate solution Diaminobenzidine (DAB) for 5 min to visualize the positive bands.
Immunohistochemistry analysis Hepatic tissue samples were fixed in 10% neutral buffered formalin, paraffin-embedded and sectioned. Briefly paraffin-embedded sections were blocked with 3% hydrogen peroxide (H2O2) for 10 min at 37 °C and washed with PBS. Subsequently, the sections were incubated in the X protein mouse monoclonal antibody (diluted 1:100) for 2hr at 37 °C. After washing with PBS, the sections were incubated in horseradish peroxidase-labeled sheep anti mouse IgG (diluted 1:50) for 40 min at 37 °C. Washed with PBS three times, the sections were subjected to color reaction with 0.02% 3,3-diaminobenzidine tetrahydrochloride containing 0.005% H2O2 in PBS and counterstained with hematoxylin lightly.
Immunogold transmission electron microscopy The immunohistochemical X protein-positive mouse liver tissue was selected to be cut into small pieces (0.1 cm in diameter) and fixed first in 2% paraformaldehyde and 0.5% glularaldehyde mixture buffer for 2hr at 4 °C, washed three times with PBS, acted upon by 0.25% Triton X-100 for 10 min. After being blocked with blocking buffter, the pieces were incubated with X protien mouse monoclonal antibody over night at 4 °C, washed with TBS and incubated with avidin-gold (15 nm) for 2hr at room temperature; then postfixed in 1% osmiam tetroxide for 1hr at room temperature, dehydrated in gradient ethanol and embedded in epoxy resin. The sections were cut on an LKB Ultralome III, mounted on copper grids, stained with uranyl acetate and led citrate, and examined by transmission electron mocroscopy.
A 0.465 kb HBx gene was amplificated from HBV genomic DNA and subcloned into the expression vector pcDNA3, with which the pcDNA3-HBx was constructed. The sequence of HBx gene in the plasmid was coincident with that reported befoer[3], as identified by restriction endonucleases digestion and confined by DNA direct sequencing. After purification by gel extraction, pcDNA3-HBx plasmids were transfected into Hela cells. Positive clones, Hela-HBx cells were isolated by G418 selection. With Western blotting, hepatitis X protein was detected in Hela cells, suggesting pcDNA3-HBx plasmids expressed in eukaryotic cells (Figure 1).
The pcDNA3-HBx plasmid was digested by Sal I and target fragments containing CMV promoter and HBx ORF were purified by gel extraction. Target fragments then were microinjected into male pronuclei of zygotes from C57BL/6 mice. 45 zygotes were microopreated. 41 microinjected eggs were implanted into oviducts of 3 pseudopregnant recipient mice, and 4 pups were born and survived. The born rate was 11%. By multiplex PCR screening, two of the pups were identified to harbour HBx gene in their genomic DNA, named C57-TgN HMU1 and C57-TgN (HBx) SMMU3 (Figure 2).
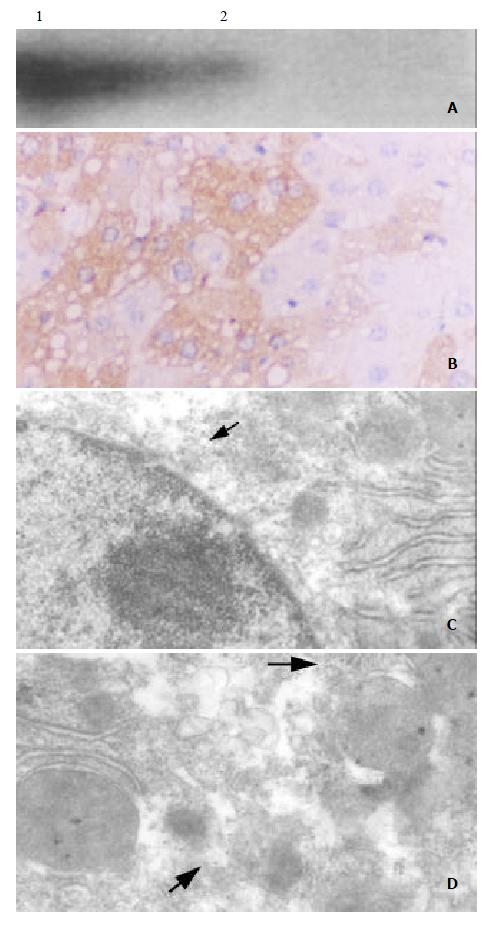
To detect the expression of hepatitis X protein in transgenic mice, liver samples were obtained from C57-TgN(HBx) SMMU1 mice and normal C57BL/6 mice. Specimens were homogenized and lyzed in lysis buffer. 100 mg lysate was used to assay HBX protein. A component of relative molecular mass 17000 befitting the X protein was specifically detected with anti X protein monoantibody by Western blotting (Figure 3A), suggesting the transgenic mice with HBx gene could express X protein in the liver tissue. The distribution of X protein in hepacytes was determined by immunohistochemisty and immunogold electron microscopy, which revealed that X protein was mainly distributed in hepatocytic cytoplasma, little on plasm membrane and in nucleus (Figure 3B, Figure 3C, Figure 3D).
Transgenic mice are the valueble animal models to study the functions of genes[30]. Although transgenic mice containing different HBV genes, including the entire viral genome, have been established and analysed before, there is little evidence to suggest that the virus plays a direct role in inducing hepatocellular carcinoma[31-41]. Hepatitis X protein is essential for HBV genes expression and replication[42,43]. In vitro, X protein exhibits a plethora of activities. From cell culture studies, it is believed that X protein can activate the transcription of host genes, including the major histocompatibility complex and c-myc, as well as viral genes. Aside from the transactivation of many promoters, the other activities linked to X protein include stimulation of signal transduction and binding, to various degrees, to well-known protein targets such as p53, proteasome subunit, and UV-damaged DNA binding protein[44-58].
However, the role of HBx gene in the course of HBV infection and in inducing HCC is unknown. In the present study, we constructed an HBx gene (adr subtype) expression vector pcDNA3-HBx containing CMV promoter and HBx gene ORF. By Western blotting, we found that it could express X protein in eukaryotic cells. pcDNA3-HBx may be a useful vector to study the role of X protein and explore the machansim of transactivation in vitro. We also generated two founders of transgenic mice with HBx gene(adr subtype) by microinjections, named C57-TgN (HBx) SMMU1 and C57-TgN(HBx)SMMU3, which harboured HBx gene in their genomic DNA. The birth rate of the pups was lower than that of other transgenic mice, including entire hepatitis viral genome transgenic mice. This indicated that X protein was probably involved in some phases of development. The hepatitis X protein was expressed in the liver tissue of transgenic mice and distributed mainly in hepacytes cytoplasm by Western blotting, immunohistochemisty and immunogold electron microscopy, which suggested that the transgenic mice could be an important tool in studying the function of HBx gene in vivo. Besides, we also developed a multiplex PCR to rapidly and accurately screen the transgenic mice with HBx gene. This method, using an optimized ratio of primer pairs, allows for the detection of HBx gene in transgenic mice, which can not only amplificate target genes, but also show its amplification efficiency.
In conclusion, we have established HBx (adr subtype) transgenic mice as a model systerm for defining the function of HBx gene (adr subtype) and the role of X protein in the virus life cycle and HCC. And the multiplex PCR is a rapidly and accurately method to detect the transgenic mice with HBx gene.
Edited by Zhu L
| 1. | Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature. 1985;317:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 928] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 2. | Ganem D, Varmus HE. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 827] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 3. | Gan RB, Chu MJ, Shen LP, Qian SW, Li ZP. The complete nucleotide sequence of the cloned DNA of hepatitis B virus subtype adr in pADR-1. Sci Sin B. 1987;30:507-521. [PubMed] |
| 4. | Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1115] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 5. | Stuyver LJ, Locarnini SA, Lok A, Richman DD, Carman WF, Dienstag JL, Schinazi RF. Nomenclature for antiviral-resistant human hepatitis B virus mutations in the polymerase region. Hepatology. 2001;33:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 298] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Wu X, Zhu L, Li ZP, Koshy R, Wang Y. Functional organization of enhancer (ENII) of hepatitis B virus. Virology. 1992;191:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1202] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 8. | Chisari FV. Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am J Pathol. 2000;156:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 229] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Montalto G, Cervello M, Giannitrapani L, Dantona F, Terranova A, Castagnetta LA. Epidemiology, risk factors, and natural history of hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Arbuthnot P, Kew M. Hepatitis B virus and hepatocellular carcinoma. Int J Exp Pathol. 2001;82:77-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Honda M, Kaneko S, Kawai H, Shirota Y, Kobayashi K. Differential gene expression between chronic hepatitis B and C hepatic lesion. Gastroenterology. 2001;120:955-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Liu H, Wang Y, Zhou Q, Gui SY, Li X. The point mutation of p53 gene exon7 in hepatocellular carcinoma from Anhui Province, a non HCC prevalent area in China. World J Gastroenterol. 2002;8:480-482. [PubMed] |
| 13. | Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, Chen J, Gao DM, Bao WH. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7:630-636. [PubMed] |
| 14. | Cao XY, Liu J, Lian ZR, Clayton M, Hu JL, Zhu MH, Fan DM, Feitelson M. Differentially expressed genes in hepatocellular carcinoma induced by woodchuck hepatitis B virus in mice. World J Gastroenterol. 2001;7:575-578. [PubMed] |
| 15. | Lee JH, Ku JL, Park YJ, Lee KU, Kim WH, Park JG. Establishment and characterization of four human hepatocellular carcinoma cell lines containing hepatitis B virus DNA. World J Gastroenterol. 1999;5:289-295. [PubMed] |
| 16. | Madden CR, Finegold MJ, Slagle BL. Hepatitis B virus X protein acts as a tumor promoter in development of diethylnitrosamine-induced preneoplastic lesions. J Virol. 2001;75:3851-3858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Koike K, Moriya K, Yotsuyanagi H, Iino S, Kurokawa K. Induction of cell cycle progression by hepatitis B virus HBx gene expression in quiescent mouse fibroblasts. J Clin Invest. 1994;94:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Shih WL, Kuo ML, Chuang SE, Cheng AL, Doong SL. Hepatitis B virus X protein inhibits transforming growth factor-beta -induced apoptosis through the activation of phosphatidylinositol 3-kinase pathway. J Biol Chem. 2000;275:25858-25864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Benn J, Schneider RJ. Hepatitis B virus HBx protein deregulates cell cycle checkpoint controls. Proc Natl Acad Sci USA. 1995;92:11215-11219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 236] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Ogden SK, Lee KC, Barton MC. Hepatitis B viral transactivator HBx alleviates p53-mediated repression of alpha-fetoprotein gene expression. J Biol Chem. 2000;275:27806-27814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Su F, Theodosis CN, Schneider RJ. Role of NF-kappaB and myc proteins in apoptosis induced by hepatitis B virus HBx protein. J Virol. 2001;75:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Diao J, Garces R, Richardson CD. X protein of hepatitis B virus modulates cytokine and growth factor related signal transduction pathways during the course of viral infections and hepatocarcinogenesis. Cytokine Growth Factor Rev. 2001;12:189-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Kaneko S, Miller RH. X-region-specific transcript in mammalian hepatitis B virus-infected liver. J Virol. 1988;62:3979-3984. [PubMed] |
| 24. | Terradillos O, Pollicino T, Lecoeur H, Tripodi M, Gougeon ML, Tiollais P, Buendia MA. p53-independent apoptotic effects of the hepatitis B virus HBx protein in vivo and in vitro. Oncogene. 1998;17:2115-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor alpha. Proc Natl Acad Sci USA. 1997;94:8744-8749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 251] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol. 2001;36:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Feitelson MA, Duan LX. Hepatitis B virus X antigen in the pathogenesis of chronic infections and the development of hepatocellular carcinoma. Am J Pathol. 1997;150:1141-1157. [PubMed] |
| 28. | Miyatake S, Yokota T, Lee F, Arai K. Structure of the chromosomal gene for murine interleukin 3. Proc Natl Acad Sci USA. 1985;82:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Lambert JF, Benoit BO, Colvin GA, Carlson J, Delville Y, Quesenberry PJ. Quick sex determination of mouse fetuses. J Neurosci Methods. 2000;95:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Rall GF, Lawrence DM, Patterson CE. The application of transgenic and knockout mouse technology for the study of viral pathogenesis. Virology. 2000;271:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Koike K. Hepatocarcinogenesis in hepatitis viral infection: lessons from transgenic mouse studies. J Gastroenterol. 2002;37 Suppl 13:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Chen XS, Wang GJ, Cai X, Yu HY, Hu YP. Inhibition of hepatitis B virus by oxymatrine in vivo. World J Gastroenterol. 2001;7:49-52. [PubMed] |
| 33. | Aragona E, Burk RD, Ott M, Shafritz DA, Gupta S. Cell type-specific mechanisms regulate hepatitis B virus transgene expression in liver and other organs. J Pathol. 1996;180:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Xu Z, Yen TS, Wu L, Madden CR, Tan W, Slagle BL, Ou JH. Enhancement of hepatitis B virus replication by its X protein in transgenic mice. J Virol. 2002;76:2579-2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158-6169. [PubMed] |
| 36. | Zhu HZ, Cheng GX, Chen JQ, Kuang SY, Cheng Y, Zhang XL, Li HD, Xu SF, Shi JQ, Qian GS. Preliminary study on the production of transgenic mice harboring hepatitis B virus X gene. World J Gastroenterol. 1998;4:536-539. [PubMed] |
| 37. | Chemin I, Ohgaki H, Chisari FV, Wild CP. Altered expression of hepatic carcinogen metabolizing enzymes with liver injury in HBV transgenic mouse lineages expressing various amounts of hepatitis B surface antigen. Liver. 1999;19:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Schweizer J, Valenza-Schaerly P, Goret F, Pourcel C. Control of expression and methylation of a hepatitis B virus transgene by strain-specific modifiers. DNA Cell Biol. 1998;17:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 868] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 40. | Hu YP, Hu WJ, Zheng WC, Li JX, Dai DS, Wang XM, Zhang SZ, Yu HY, Sun W, Hao GR. Establishment of transgenic mouse harboring hepatitis B virus (adr subtype) genomes. World J Gastroenterol. 2001;7:111-114. [PubMed] |
| 41. | Hu YP, Yao YC, Li JX, Wang XM, Li H, Wang ZH, Lei ZH. The cloning of 3'-truncated preS/S gene from HBV genomic DNA and its expression in transgenic mice. World J Gastroenterol. 2000;6:734-737. [PubMed] |
| 42. | Ganem D. Virology. The X files--one step closer to closure. Science. 2001;294:2299-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Bouchard MJ, Wang LH, Schneider RJ. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001;294:2376-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 327] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 44. | Yun C, Um HR, Jin YH, Wang JH, Lee MO, Park S, Lee JH, Cho H. NF-kappaB activation by hepatitis B virus X (HBx) protein shifts the cellular fate toward survival. Cancer Lett. 2002;184:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Bergametti F, Sitterlin D, Transy C. Turnover of hepatitis B virus X protein is regulated by damaged DNA-binding complex. J Virol. 2002;76:6495-6501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Li J, Xu Z, Zheng Y, Johnson DL, Ou JH. Regulation of hepatocyte nuclear factor 1 activity by wild-type and mutant hepatitis B virus X proteins. J Virol. 2002;76:5875-5881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Han HJ, Jung EY, Lee WJ, Jang KL. Cooperative repression of cyclin-dependent kinase inhibitor p21 gene expression by hepatitis B virus X protein and hepatitis C virus core protein. FEBS Lett. 2002;518:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Wang XZ, Jiang XR, Chen XC, Chen ZX, Li D, Lin JY, Tao QM. Seek protein which can interact with hepatitis B virus X protein from human liver cDNA library by yeast two-hybrid system. World J Gastroenterol. 2002;8:95-98. [PubMed] |
| 49. | Guo SP, Wang WL, Zhai YQ, Zhao YL. Expression of nuclear factor-kappa B in hepatocellular carcinoma and its relation with the X protein of hepatitis B virus. World J Gastroenterol. 2001;7:340-344. [PubMed] |
| 50. | Lee S, Tarn C, Wang WH, Chen S, Hullinger RL, Andrisani OM. Hepatitis B virus X protein differentially regulates cell cycle progression in X-transforming versus nontransforming hepatocyte (AML12) cell lines. J Biol Chem. 2002;277:8730-8740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Qiao L, Leach K, McKinstry R, Gilfor D, Yacoub A, Park JS, Grant S, Hylemon PB, Fisher PB, Dent P. Hepatitis B virus X protein increases expression of p21(Cip-1/WAF1/MDA6) and p27(Kip-1) in primary mouse hepatocytes, leading to reduced cell cycle progression. Hepatology. 2001;34:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Waris G, Huh KW, Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol. 2001;21:7721-7730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 278] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 53. | Nag A, Datta A, Yoo K, Bhattacharyya D, Chakrabortty A, Wang X, Slagle BL, Costa RH, Raychaudhuri P. DDB2 induces nuclear accumulation of the hepatitis B virus X protein independently of binding to DDB1. J Virol. 2001;75:10383-10392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Lin-Marq N, Bontron S, Leupin O, Strubin M. Hepatitis B virus X protein interferes with cell viability through interaction with the p127-kDa UV-damaged DNA-binding protein. Virology. 2001;287:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Tarn C, Lee S, Hu Y, Ashendel C, Andrisani OM. Hepatitis B virus X protein differentially activates RAS-RAF-MAPK and JNK pathways in X-transforming versus non-transforming AML12 hepatocytes. J Biol Chem. 2001;276:34671-34680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 56. | Jaitovich-Groisman I, Benlimame N, Slagle BL, Perez MH, Alpert L, Song DJ, Fotouhi-Ardakani N, Galipeau J, Alaoui-Jamali MA. Transcriptional regulation of the TFIIH transcription repair components XPB and XPD by the hepatitis B virus x protein in liver cells and transgenic liver tissue. J Biol Chem. 2001;276:14124-14132. [PubMed] |
| 57. | Pan J, Duan LX, Sun BS, Feitelson MA. Hepatitis B virus X protein protects against anti-Fas-mediated apoptosis in human liver cells by inducing NF-kappa B. J Gen Virol. 2001;82:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Sitterlin D, Bergametti F, Transy C. UVDDB p127-binding modulates activities and intracellular distribution of hepatitis B virus X protein. Oncogene. 2000;19:4417-4426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |